|
|
| | 3,6-DIOXA-1,8-OCTANEDITHIOL Basic information |
| Product Name: | 3,6-DIOXA-1,8-OCTANEDITHIOL | | Synonyms: | Triglycoldimercaptan;ETHYLENE GLYCOL BIS(2-MERCAPTOETHYL) ETHER;1,2-BIS(2-MERCAPTOETHOXY)ETHANE;3,6-DIOXA-1,8-OCTANEDITHIOL;Triethyleneglycol dimrcaptan;Ethanethiol, 2,2-1,2-ethanediylbis(oxy)bis-;2,2′-[1,2-Ethandiylbis(oxy)]bis(ethanthiol);2,2'-[1,2-ethanediylbis(oxy)]bis-Ethanethiol Triglycol dimercaptan 2,2'-[1,2-ethanediylbis(oxy)]bis-ethanethio 2,2'-(ethylenedioxy)diethanethiol | | CAS: | 14970-87-7 | | MF: | C6H14O2S2 | | MW: | 182.3 | | EINECS: | 239-044-2 | | Product Categories: | peg | | Mol File: | 14970-87-7.mol | 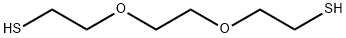 |
| | 3,6-DIOXA-1,8-OCTANEDITHIOL Chemical Properties |
| Boiling point | 225 °C(lit.) | | density | 1.12 g/mL at 25 °C(lit.) | | vapor pressure | 0.39Pa at 20℃ | | refractive index | n20/D 1.509(lit.) | | Fp | 132 °C | | solubility | Slightly soluble in water | | form | clear liquid | | pka | 9.34±0.10(Predicted) | | color | Colorless to Light yellow to Light orange | | Water Solubility | 11.4g/L at 19.8℃ | | BRN | 1901671 | | InChI | InChI=1S/C6H14O2S2/c9-5-3-7-1-2-8-4-6-10/h9-10H,1-6H2 | | InChIKey | HCZMHWVFVZAHCR-UHFFFAOYSA-N | | SMILES | C(OCCS)COCCS | | LogP | 1.6 at 55℃ | | CAS DataBase Reference | 14970-87-7(CAS DataBase Reference) | | EPA Substance Registry System | Ethanethiol, 2,2'-[1,2-ethanediylbis(oxy)]bis- (14970-87-7) |
| | 3,6-DIOXA-1,8-OCTANEDITHIOL Usage And Synthesis |
| Uses | 3,6-dioxa-1,8-octanedithiol (DODT) was used for cleaving peptides in the study to develop synthetic peptide serology to treat chronic chagas disease. DODT may be used to develop non-malodorous scavenger in Fmoc-based peptide synthesis. | | Definition | 3,6-dioxa-1,8-octanedithiol is a dithiol monomer. This compound could be chosen as a model compound for the active sites of thioredoxins to study its reactions with cis-[Pt(NH3)2Cl4] and trans-[PtCl2(CN)4]2– (cisplatin prodrug and a model complex). The pKa values for the dithiol were characterized to be 8.7 ± 0.2 and 9.6 ± 0.2 at 25.0 °C and an ionic strength of 1.0 M. Triethylamine (TEA) and dilute H2O2 could catalyze the new Radical Ring-opening Redox Polymerization (R3P) of 3,6-dioxa-1,8-octanedithiol[1-2]. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 33, p. 1275, 1968 DOI: 10.1021/jo01267a089
Triglycol dimercaptan | | General Description | 3,6-dioxa-1,8-octanedithiol is non volatile in nature. | | References | [1] DONGYING MA. Formation of peptide disulfide bonds through a trans-dibromido-Pt(IV) complex oxidation reaction: Kinetic and mechanistic analyses[J]. Journal of Molecular Liquids, 2021. DOI:10.1016/j.molliq.2020.115195.
[2] ROSENTHAL-KIMEMILY Q PuskasJudit E. Green polymer chemistry: investigating the mechanism of radical ring-opening redox polymerization (R3P) of 3,6-dioxa-1,8-octanedithiol (DODT).[J]. Molecules, 2015. DOI:10.3390/molecules20046504. |
| | 3,6-DIOXA-1,8-OCTANEDITHIOL Preparation Products And Raw materials |
|