- Procymidone
-

- $0.00 / 25kg
-
2025-04-11
- CAS:32809-16-8
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 20tons
- Procymidone
-

- $100.00 / 50kg
-
2025-04-01
- CAS:32809-16-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton
- Procymidone
-
- $1.00 / 1KG
-
2024-08-02
- CAS:32809-16-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
|
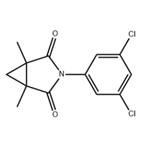
| Product Name: | Procymidone | | Synonyms: | 1,2-Cyclopropanedicarboximide, N-(3,5-dichlorophenyl)-1,2-dimethyl-;1,2-dimethyl-N-(3,5-dichlorophenyl)cyclopropanedicarboximide;3-(3,5-dichlorophenyl)-1,5-dimethyl-3-azabicyclo(3.1.0)hexane-4-dione;3-azabicyclo[3.1.0]hexane-2,4-dione,3-(3,5-dichlorophenyl)-1,5-dimethyl-;DICYCLIDINE;procymidone (bsi,iso,jmaf);PROCYMIDONE(BSI,ISO);3-(3,5-dichlorophenyl)-1,5-dimethyl-3-azabicyclo[3.1.0]hexane-2,4-dione | | CAS: | 32809-16-8 | | MF: | C13H11Cl2NO2 | | MW: | 284.14 | | EINECS: | 251-233-1 | | Product Categories: | FUNGICIDE;DicarboximidesPesticides&Metabolites;Alpha sort;Fungicides;N-PAlphabetic;P;Pesticides;PON - PT;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 32809-16-8.mol | 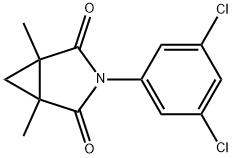 |
| | Procymidone Chemical Properties |
| WGK Germany | 2 | | RTECS | GZ2150000 | | HS Code | 29251900 | | Toxicity | LD50 in male rats (mg/kg): 6800 orally, >10000 dermally (Jackson); LD50 in male, female rats (g/kg): 7.8, 9.1 orally (Mikami) |
| | Procymidone Usage And Synthesis |
| Description | Procymidone is a moderately systemic fungicide. It is used in horticulature as a seed-dressing, pre-harvest spray or post-harvest dip for the control of various fungal diseases, such as grey mold, neck rot, brown rot, blossom blight, and twig wilt. It is applied to vegetables including onions, fruits (including peaches, plums, grapes, stone fruit, strawberries, and cherries), and ornamentals.
| | Uses | Systemic agricultural fungicide. | | Uses | Procymidone is a systemic fungcide with both protective and
curative activities used to control plant diseases such as fruit rots, grey
mould on top fruits, vines and vegetables, and Sclerotinia rot of kidney
beans and vegetable crops. | | Definition | ChEBI: An azabicycloalkane that is 1,5-dimethyl-3-azabicyclo[3.1.0]hexane-2,4-dione in which the amino hydrogen is replaced by a 3,5-dichlorophenyl group. A fungicide widely used in horticulture as a seed dressing, pre-harvest spray or post-harvest dip for the co
trol of various diseases. | | Metabolic pathway | The fungicides, chlozolinate, vinclozolin, and
procymidone, are added to wine after fermentation
and the degradation products are isolated and
identified. Chlozolinate undergoes a rapid hydrolytic
loss of the ethoxycarbonyl substituent to give an
oxazolidine that further undergoes hydrolytic cleavage
to give 3' ,5' -dichloro-2-hydroxypropanilide. The
oxazolidine ring of vinclozolin undergoes a similar
hydrolysis reaction to give the corresponding anilide, 3' ,5'-dichloro-2-hydroxy-2-methylbut-3-eneanilide. Both
of these anilides are stable in wine for 150 days. A
different degradation behavior is observed with
procymidone and leads to the formation of 3,5-
dichloroaniline, which, in turn, breaks down in wine. | | Degradation | Procymidone (1) was stable in acidic conditions (pH 2) but hydrolysed
rapidly in buffer solutions (pH 6 to 10) and in natural river and sea water
at 15, 30 and 45 °C with DTm values from 30 min to 8 days. Degradation
occurred mainly via cleavage of the cyclic imide linkage in alkaline
conditions and via cleavage of the subsequent amide linkage in acidic
conditions (Mikami and Miyamoto, 1981) to yield the intermediate acid
[2-( 3,5-dichlorophenylcarbamoyl)- 1,2 -dimet hylcyclopropanecarboxylic
acid (2) and 1,2-dimethylcyclopropane-1,2-dicarboxylica cid (3) and 3,5-
dichloroaniline (4) as terminal products (Villedieu et al., 1994,1995). Pirisi
et al. (1986) and Cabras et al. (1984) reported the formation of compound 4
and other polar decomposition products in wine during the fermentation
process.
Photodegradation of procymidone was investigated in various
solutions after exposure to sunlight. Major degradation pathways were
cleavage of the cyclic imide and the subsequent cleavage of the amide
linkage to yield compounds 3 and 4 (Mikami and Miyamoto, 1981). |
| | Procymidone Preparation Products And Raw materials |
|