| Company Name: |
Ereztech LLC
|
| Tel: |
1.888.658.1221 |
| Email: |
customerservice@ereztech.com |
| Products Intro: |
Product Name:Triiron dodecacarbonyl
CAS:12088-65-2
|
|
| | IRON DODECACARBONYL Basic information |
| Product Name: | IRON DODECACARBONYL | | Synonyms: | IRON DODECACARBONYL;IRON DODECARBONYL;TRIIRON(0) DODECACARBONYL;TRIIRON DODECACARBONYL;5-10%methanolstabilized | | CAS: | 12088-65-2 | | MF: | C12Fe3O12 | | MW: | 503.66 | | EINECS: | 241-668-5 | | Product Categories: | | | Mol File: | 12088-65-2.mol | 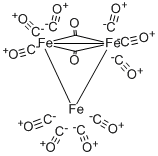 |
| | IRON DODECACARBONYL Chemical Properties |
| Melting point | 165 °C (dec.)(lit.) | | density | 2.0000 | | storage temp. | 2-8°C | | form | black crystals | | color | dark green crystals, crystalline |
| | IRON DODECACARBONYL Usage And Synthesis |
| Chemical Properties | stabilized with 5%–10% methanol; black crystal(s); sensitive to air [STR93] | | Chemical Properties | Tri-iron dodecacarbonyl
forms deep green, diamagnetic crystals which decompose at 140°C, but which can be sublimed
slowly in vacuo. It dissolves in organic solvents and in pentacarbonyl iron in which
its molecular weight corresponds to Fe3(CO)12. | | Preparation | Tri-iron Dodecacarbonyl was first obtained by thermal decomposition of non aqueous solutions
of the enneacarbonyl at 100°C :
6Fe2(CO)9 -> 2Fe3(CO)12+6Fe(CO)5
but is now usually prepared from solutions of carbonylferrate anions. One method uses
the oxidation of these ions with manganese(IV) oxide followed by acidification and extraction
of the carbonyl into petroleum ether. In another method,iron pentacarbonyl
is treated with aqueous triethylamine to form the dark red [Et3NH][Fe3(CO)11H] which is
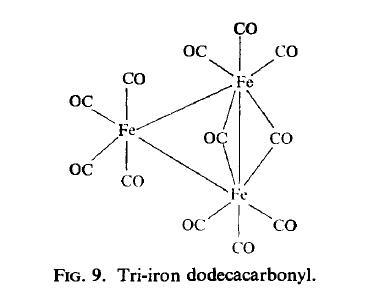
then acidified and the carbonyl extracted into petroleum ether. | | Structure and conformation | The structure of Fe3(CO)12 (Fig.9) is related to that of Fe2(CO)9 in that it can be
described as a triangular metal cluster in which one bridging group of the Fe2(CO)9 system is replaced by an Fe(CO)4 group. This X-ray data is in agreement with the M?ssbauer
spectrum which shows the presence of two types of iron atom ; two of the iron atoms
show substantial quadrupole splitting and have isomer shifts similar to those of the iron
atoms in Fe2(CO)9, while the other iron atom shows little splitting as might be expected for
an octahedral environment. A similar structure is possessed by the [Fe3(CO)11H] anion in
which one of the bridging carbonyl groups is replaced by a bridging hydrogen atom.
 |
| | IRON DODECACARBONYL Preparation Products And Raw materials |
|