- GSK 3 Inhibitor IX
-

- $37.00 / 1mg
-
2024-11-20
- CAS:667463-62-9
- Min. Order:
- Purity: 99.72%
- Supply Ability: 10g
- BIO
-

- $1.00 / 1KG
-
2019-07-07
- CAS:667463-62-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg
|
| Product Name: | BIO | | Synonyms: | 2H-Indol-2-one, 6-broMo-3-[(3E)-1,3-dihydro-3-(hydroxyiMino)-2H-indol-2-ylidene]-1,3-dihydro-, (3Z)-;6BIO;6-bromoindirubin-3-oxime;GSK3 Inhibitor IX1;BIO(GSK-3 Inhibitor IX;BIO (SM04554);InSolution? GSK-3 Inhibitor IX;(2E,3E)-6'-Bromo-3-(hydroxyimino)-[2,3'-biindolinylidene]-2'-one | | CAS: | 667463-62-9 | | MF: | C16H10BrN3O2 | | MW: | 356.17 | | EINECS: | | | Product Categories: | Inhibitors;Intracellular Signaling;Akt;mTOR;PI3K | | Mol File: | 667463-62-9.mol | 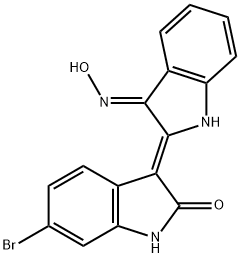 |
| Melting point | 300°C(lit.) | | Boiling point | 554.3±50.0 °C(Predicted) | | density | 1.80±0.1 g/cm3(Predicted) | | RTECS | NM3241900 | | storage temp. | 2-8°C | | solubility | DMSO: >5 mg/mL | | form | solid | | pka | 9.56±0.20(Predicted) | | color | dark red | | Sensitive | Air & Light Sensitive | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | HS Code | 29339900 |
| Description | BIO is a cell-permeable bis-indolo (indirubin) compound that acts as a highly potent, selective, reversible, and ATP-competitive inhibitor of GSK3α/β (IC50 = 5 nM). Its specificity has been tested against various cyclin-dependent kinases (CDKs) (IC50s = 83, 300, 320, and 10,000 nM for Cdk5/p25, Cdk2/A, Cdk1/B, and Cdk4/D1, respectively). BIO was also tested for its specificity towards many other commonly studied kinases (IC50 ≥10 μM), including MAP kinases, PKA, PKC isoforms, PKG, CK, and IRTK. Inhibition of GSK by BIO has been shown to result in the activation of the Wnt signaling pathway and sustained pluripotency in human and mouse embryonic stem cells (ESCs). BIO is reported to maintain self-renewal in human and mouse ESCs as well as induce the differentiation of neonatal cardiomyocytes. | | Uses | 6-bromoindirubin-3′-oxime (BIO) has been used:
- in MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) cell proliferation assay
- as a medium supplement in embryonic stem cells (ESCs)
- for the inhibition of glycogen synthase kinase 3 β (GSK-3β) in human dermal papilla cells (hDPCs)
| | Definition | ChEBI: 6-bromoindirubin-3'-oxime is a member of the class of biindoles that is indirubin substituted at position 6 by a bromo group and in which the keto group at position 3' has undergone condensation with hydroxylamine to form the corresponding oxime. It has a role as an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor and an EC 2.7.11.26 (tau-protein kinase) inhibitor. It is a ketoxime, an organobromine compound, a member of oxindoles and a biindole. | | General Description | A cell-permeable, highly potent, selective, reversible, and ATP-competitive inhibitor of GSK-3α/β (IC50 = 5 nM). Specificity was confirmed using various Cdk′s (IC50 = 83 nM for Cdk5/p25, 300 nM for Cdk2/cyclin A, 320 nM for Cdk1/cyclin B, and 10 μM for Cdk4/cyclin D1), MAP kinases, PKA, PKC isoforms, PKG, CK, and IRTK (IC50 ≥ 10 μM). Inhibition of GSK by BIO has been shown to result in the activation of the Wnt signaling pathway and sustained pluripotency of human and murine embryonic stem cells (ESCs). | | Biological Activity | Potent and selective, ATP-competitive glycogen synthase kinase-3 (GSK-3) inhibitor (IC 50 values are 5, 83, 300, 320 and > 10 000 nM at GSK-3, CDK5/p25, CDK2/cyclin A, CDK1/cyclin B and other common kinases respectively). Maintains self-renewal and pluripotency in embryonic stem cells via activation of the Wnt signaling pathway in vitro . | | Biochem/physiol Actions | Cell permeable: yes | | Enzyme inhibitor | These protein kinase inhibitors (FWBIO = 356.19 g/mol; CAS 667463-62-9; Solubility: 70 mg/mL DMSO, <1mg/mL H2O), also known as BIO, 6BIO, and systematically as 6-bromo-3-[(3E)-1,3-dihydro-3-(hydroxyimino)-2Hindol- 2-ylidene]-1,3-dihydro-(3Z)-2H-indol-2-one, targets glycogen synthase kinase-3 (or GSK-3), with an IC50 value of 5 nM for α and β forms. BIO also shows greater than 16x selectivity over CDK5 and is also a weaker pan-JAK inhibitor, with IC50 values of 30 nM, 1.5 μM, 8.0 μM, and 0.5 μM for TYK2, JAK1, JAK2 and JAK3. (See the structural related inhibitor 1-Azakenpaullone) BIO, but not 1-methyl-BIO, closely mimicked Wnt signaling in Xenopus embryos. BIO-induced apoptosis of human melanoma cells is associated with reduced phosphorylation of JAKs and STAT3 in both dose- and time-dependent manners. Consistent with inhibition of STAT3 signaling, expression of the anti-apoptotic protein Mcl- 1 was down-regulated. Maintaining Embryonic Stem Cells Pluripotency: Human and mouse embryonic stem cells (ESCs) self-renew indefinitely while maintaining the ability to generate all three germ-layer derivatives. Importantly, Wnt pathway activation by BIO is sufficient to maintain the undifferentiated phenotype in both types of ESCs and sustains expression of the pluripotent state-specific transcription factors Oct-3/4, Rex-1 and Nanog. Such results suggest that BIO and related GSK-3-specific inhibitors may off practical advantages in regenerative medicine. Bio can also participate in controlling the proliferative capability of the highly differentiated cardiomyocytes. Activation of Wnt/β-catenin and inhibition of Notch signaling pathways, as mediated by simultaneous co-application of BIO and the γ-secretase inhibitor N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2- phenylglycine-1,1-dimethylethyl ester (or DAPT), efficiently induces intestinal differentiation of ESCs cultured on feeder cells. These findings that Wnt and Notch signaling function to pattern the anterior-posterior axis of the DE and control intestinal differentiation. 6-Bromoindirubin-3'- acetoxime: This cell-permeable BIO analogue (FWBIO-acetoxime = 398.21 g/mol; CAS 667463-85-6) is active against herpes simplex virus-1 (HSV-1) infection in human oral epithelial cells, the latter representing a natural target cell type. BIO-acetoxime reduces viral yields and the expression of different classes of viral proteins. BIO-acetoxime may suppress viral gene expression and protect oral epithelial cells from HSV-1 infection. Tyrian Purple: BIO’s indirubin nucleus is related to the famous Tyrian purple dye that the Phoenicians isolated from the gastropod mollusk Hexaplex trunculus and gained favor in antiquity, because it did not fade, actually becoming more brilliant upon weathering and exposure to sunlight. The inhibitory properties of BIO suggest that 6-bromoindirubin provides a new scaffold for the development of selective and potent pharmacological inhibitors of GSK- 3. | | in vivo | study showed bio activated maternal wnt signaling pathway in xenopus embryos. it caused a hyper dorso-anteriorization at the expense of trunk and tail in a dose-response manner. it also activated the dorsal genes (siamois and chordin) ectopically. [1] | | storage | +4°C | | References | 1) Meijer et al. (2003), GSK-3-selective inhibitors derived from Tyrian purple indirubins; Chem. Biol., 10 1255
2) Tseng et al. (2006), The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes; Chem. Biol., 13 957
3) Chebel et al. (2009), Indirubin derivatives inhibit malignant lymphoid cell proliferation; Leuk. Lymphoma, 50 2049 |
| | BIO Preparation Products And Raw materials |
|