- Pyrogallol
-

- $0.00 / 25kg
-
2025-04-07
- CAS:87-66-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000mt/year
- Pyrogallol
-

- $6.00 / 1kg
-
2025-04-07
- CAS:87-66-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- Pyrogallol
-

- $0.00 / 1kg
-
2025-04-07
- CAS:87-66-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
|
| | Pyrogallol Basic information |
| Product Name: | Pyrogallol | | Synonyms: | 1,2,3-TRIHYDROXYBENZENE TRIMETHYL ETHER;1,2,3-BENZENETRIOL TRIMETHYL ETHER;1,2,3-Trihydroxybenene;Pyrogallic acid~1,2,3-Trihydroxybenzene;Pyrogallol solution;PYROGALLOL REAGENT (ACS);PYROGALLOL,CRYSTAL,REAGENT,ACS;1,2,3-trihydroxy-benzen | | CAS: | 87-66-1 | | MF: | C6H6O3 | | MW: | 126.11 | | EINECS: | 201-762-9 | | Product Categories: | ACS Grade;Building Blocks;Chemical Synthesis;Essential Chemicals;Inorganic Salts;Organic Building Blocks;Oxygen Compounds;FINE Chemical & INTERMEDIATES;Aromatic Hydrocarbons (substituted) & Derivatives;Nutrition Research;Phytochemicals by Plant (Food/Spice/Herb);s wort);Pharmacopoeia;Pharmacopoeia A-Z;Food additive , Antioxidant anticorrosive;Polyols;Research Essentials;Solutions and Reagents;87-66-1 | | Mol File: | 87-66-1.mol | 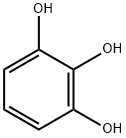 |
| | Pyrogallol Chemical Properties |
| Melting point | 43-47 °C(lit.) | | Boiling point | 309 °C | | density | 1.112 g/mL at 25 °C(lit.) | | bulk density | 600kg/m3 | | vapor density | 4.4 (vs air) | | vapor pressure | 10 mm Hg ( 167.7 °C) | | refractive index | n20/D 1.387 | | Fp | >230 °F | | storage temp. | Store below +30°C. | | solubility | water: soluble | | form | Very Fine Crystalline Powder | | pka | pK1:9.03(0);pK2:11.63(+1) (25°C) | | color | White | | PH | 4-5 (50g/l, H2O, 20℃) | | Water Solubility | 400 g/L (25 ºC) | | Sensitive | Light Sensitive | | Merck | 14,8000 | | BRN | 907431 | | Stability: | Stable, but decolourises in light. Combustible. Incompatible with strong oxidising agents, alkalies, metal oxides, ammonia, antipyrine, phenol, iodine, lime water, menthol, potassium permanganate, strong bases. | | InChIKey | WQGWDDDVZFFDIG-UHFFFAOYSA-N | | LogP | -0.47 | | CAS DataBase Reference | 87-66-1(CAS DataBase Reference) | | NIST Chemistry Reference | 1,2,3-Benzenetriol(87-66-1) | | EPA Substance Registry System | Pyrogallol (87-66-1) |
| | Pyrogallol Usage And Synthesis |
| Description | Pyrogallol is a natural oxidant that can generate superoxide (O2-) in alkaline solutions through autoxidation to a semiquinone radical. Importantly, the semiquinone radical can react with O2- in an acidic environment to produce a quinone and H2O2. Pyrogallol autoxidation is used in superoxide dismutase activity assays. It can also be used in assays to assess antioxidant capacity. Pyrogallol is used in some biological systems as an O2- scavenger. In other biological systems, it is used as an O2- generator. Pyrogallol effectively scavenges DPPH radical and ABTS+ in vitro. Pyrogallol is a product of tannin degradation to gallic acid by ruminant microbes and has hepatotoxic and nephrotoxic effects in vivo. | | Chemical Properties | white crystalline solid | | Chemical Properties | White or nearly white needle- or
leaf-shaped crystals or crystalline powder.Pyrogallol is practically odorless. | | Uses | Complexing agent; reducing agent; alkaline solution indicator for gaseous oxygen. | | Uses | Pyrogallol is used in the manufacture of various dyes; in dyeing furs, hairs, and feathers; for staining leather; in engraving;as a developer in photography; and as an analytical reagent.. | | Uses | Pyrogallol possesses importance as a spectrophotometric reagent in the determination of niobium and tantalum. The absorptions of niobium and tantalum complexes are usually measured at 340 and 335 nm, respectively. The niobium complex is formed in slightly acidic medium, and the tantalum complex in strongly acidic medium (4 N HC1). The absorption spectra are pH-dependen. | | Production Methods | Pyrogallol is prepared by heating dried gallic acid at about
200°C with the loss of carbon dioxide or by the
chlorination of cyclohexanol to tetrachlorocyclohexanone,
followed by hydrolysis. | | Definition | ChEBI: A benzenetriol carrying hydroxy groups at positions 1, 2 and 3. | | General Description | Odorless white to gray solid. Sinks and mixes with water. | | Air & Water Reactions | Turns gray on exposure to light or air. Water soluble. | | Reactivity Profile | Pyrogallol is a strong reducing agent. Reacts with alkalis, NH3, antipyrine, camphor, phenol, iron and lead salts, iodine, lime water, menthol and KMnO4. | | Hazard | Toxic by ingestion and skin absorption. | | Health Hazard | Inhalation of dust causes irritation of nose and throat. Ingestion may cause severe gastrointestinal irritation, convulsions, circulatory collapse, and death. Contact with eyes causes irritation. Skin contact can cause local discoloration, irritation, eczema, and death; repeated contact can cause sensitization. | | Health Hazard | The toxic symptoms are similar to those of phenol. It can enter the body by absorption through skin and ingestion. The poisoning effects are nausea, vomiting, gastritis, hemolysis, methemoglobinemia, kidney and liver damage, convulsions, and congestion of lungs. High doses can cause death. Ingestion of 2–3 g of solid can be fatal to humans. The LD50 values varied widely in species. The oral LD50 value in mice is about 300 mg/kg. | | Fire Hazard | Pyrogallol is probably combustible. | | Contact allergens | Pyrogallol belongs to the phenols group. It is an old
photograph developer and a low sensitizer in hair dyes. | | Biochem/physiol Actions | Pyrogallol also referred to as 1,2,3-trihydroxybenzene inhibits the response to nitric oxide (NO) in the rat anococcygeus muscle. | | Safety Profile | Human poison by ingestion and subcutaneous routes. An experimental poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported.
1 198 PPRSOO PYROSULFURYL CHLORIDE
Readdy absorbed through the skin. Human systemic effects by ingestion: convulsions, dyspnea, gastrointestinal effects. A severe skin and eye irritant. Incompatible with alkalies, NH3, antipyrine, phenol, iron and lead salts, iodine, KMn04. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a topical antibacterial agent, as an intermediate, hair dye component, and analytical reagent. | | Carcinogenicity | Pyrogallol was not carcinogenic
in mouse and rabbit chronic dermal studies. Mice were
treated twice weekly with pyrogallol in acetone (50%) on
the shaved flank for life. There was no increase in dermal or
systemic tumors. A similar study in rabbits also
revealed no skin tumors, although positive controls showed
an increase in tumors in both mice and rabbits.
Pyrogallol was considered to be cocarcinogenic when
administered dermally three times a week together with
the skin carcinogen benzo[a]pyrene for 440 days;
pyrogallol administered alone caused no increase in skin
tumors. |
| | Pyrogallol Preparation Products And Raw materials |
| Preparation Products | Atracurium besylate-->1,2,3-Trimethoxybenzene-->Coenzyme Q10-->1,4-BUTANEDIOL DIACRYLATE-->4-Hydroxybutyl acrylate-->7,8-DIHYDROXY-4-METHYLCOUMARIN-->Exifone-->BENDIOCARB-->1-CHLOROMETHYL-2,3,4-TRIMETHOXYBENZENE-->Gallamine triethiodide-->1,3-Benzenediol, 2,5-dimethoxy--->GALLEIN-->7,8-Dihydroxyflavone-->7,8-Dihydroxycoumarin-->2'-HYDROXY-3',4'-DIMETHOXYACETOPHENONE-->5-TERT-BUTYLPYROGALLOL-->C.I.Mordant Green 22-->GALLEIN |
|