- Prop-1-ene-1,3-sultone
-

- $180.00 / 1kg
-
2025-03-07
- CAS:21806-61-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Prop-1-ene-1,3-sultone
-

- $1.00 / 1kg
-
2025-03-07
- CAS:21806-61-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- Prop-1-ene-1,3-sultone
-

- $0.00 / 1kg
-
2025-02-12
- CAS:21806-61-1
- Min. Order: 1kg
- Purity: 99.5%min
- Supply Ability: 1000kg
|
| | Prop-1-ene-1,3-sultone Basic information |
| Product Name: | Prop-1-ene-1,3-sultone | | Synonyms: | Prop-1-ene-1,3-sultone;Prop-1-ene-1,3-sulto;1-Propene1,3-Sultone;1-Propene 1,3-Sultone >;5H-1,2-Oxathiole, 2,2-dioxide;1,3-Propene sultone;Prop-1-ene-1,3-sultone1,3-Propene Sultone;5H-oxathiole 2,2-dioxide | | CAS: | 21806-61-1 | | MF: | C3H4O3S | | MW: | 120.13 | | EINECS: | 606-834-7 | | Product Categories: | | | Mol File: | 21806-61-1.mol | 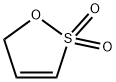 |
| | Prop-1-ene-1,3-sultone Chemical Properties |
| Melting point | 82-83 °C | | Boiling point | 257 °C | | density | 1.508 | | vapor pressure | 0.008-0.018Pa at 20-25℃ | | Fp | 109 °C | | storage temp. | 2-8°C | | solubility | soluble in Methanol | | form | powder to crystal | | color | White to Almost white | | InChI | InChI=1S/C3H4O3S/c4-7(5)3-1-2-6-7/h1,3H,2H2 | | InChIKey | KLLQVNFCMHPYGL-UHFFFAOYSA-N | | SMILES | O1CC=CS1(=O)=O | | LogP | -0.49 at 25℃ |
| | Prop-1-ene-1,3-sultone Usage And Synthesis |
| Chemical Properties | White solid | | Uses | Prop-1-ene-1,3-sultone is a chiral compound that can be used as a photoelectron sensitizer. The anion radical created by protonation or deprotonation of the ionized form of prop-1-ene-1,3-sultone can be used to generate singlet oxygen and hydrogen peroxide, which are able to oxidize organic compounds. This compound is also useful in asymmetric synthesis due to its ability to act as a ligand for transition metal ions, such as Fe2+. It has also been shown to have antioxidant properties and is soluble in both organic solvents and deionized water. | | Synthesis | A method for producing a Prop-1-ene-1,3-sultone comprises: a step 1 of chlorinating POCl3 or PCl5 with a compound of the chemical formula 2 to produce a compound of the chemical formula 3; a step 2 of diluting the compound of the chemical formula 3 and bromodan (1,3-Dibromo-5,6-dimethylhydantoin) in dichloroethylene solvent and stirring at 20-30°C for four to eight hours to produce 2-halo-1,3-propan sultone of the chemical formula 4; and a step 3 of adding base in the 2-halo-1,3-propan sultone of the chemical formula 4 and stirring for four to eight hours to produce Prop-1-ene-1,3-sultone. |
| | Prop-1-ene-1,3-sultone Preparation Products And Raw materials |
|