- DICHLOROISOCYANURIC ACID
-

- $1815.00 / 1Tons
-
2024-10-24
- CAS:2782-57-2
- Min. Order: 1Tons
- Purity: 99.99%
- Supply Ability: 100Tons
|
| | DICHLOROISOCYANURIC ACID Basic information |
| Product Name: | DICHLOROISOCYANURIC ACID | | Synonyms: | hilite60;orced;3,5-Triazine-2,4,6(1H,3H,5H)-trione,1,3-dichloro-1;5-triazine-2,4,6(1h,3h,5h)-trione,1,3-dichloro-3;6(1h,3h,5h)-trione,1,3-dichloro-s-triazine-4;acl70;cdb60;dichloro-1,3,5-triazinetrione | | CAS: | 2782-57-2 | | MF: | C3HCl2N3O3 | | MW: | 197.96 | | EINECS: | 220-487-5 | | Product Categories: | | | Mol File: | 2782-57-2.mol | 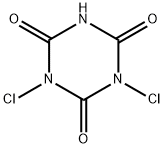 |
| | DICHLOROISOCYANURIC ACID Chemical Properties |
| Melting point | 226.4-226.7 °C | | density | 1.8400 (rough estimate) | | refractive index | 1.6100 (estimate) | | pka | 3.23±0.20(Predicted) | | Stability: | Oxidant - may ignite combustible material. | | EPA Substance Registry System | Dichloro-s-triazinetrione (2782-57-2) |
| | DICHLOROISOCYANURIC ACID Usage And Synthesis |
| Chemical Properties | White, crystalline solid, powder, or granules.
Chlorine odor. | | Chemical Properties | white crystalline powder | | Uses | Anti-infective, topical. | | General Description | Dichloroisocyanuric acid, solid is a white crystalline solid with an odor of chlorine. The material itself is noncombustible but if contaminated with a combustible material ignition can result. DICHLOROISOCYANURIC ACID will accelerate the burning of combustible materials. Contact with ammonium compounds or hydrated salts can cause a very vigorous chemical reaction. DICHLOROISOCYANURIC ACID may vigorously react with small quantities of water releasing chlorine gas. Prolonged exposure to fire or heat of the material may result in the vigorous decomposition of the material and the rupturing of its containers. Material containing less than 39 percent available chlorine will undergo reactions as described above though DICHLOROISOCYANURIC ACID may be longer to initiate and the resulting reaction may not be as vigorous. DICHLOROISOCYANURIC ACID is used as a dry bleach in household cleaning compounds and swimming pool disinfectants. | | Air & Water Reactions | DICHLOROISOCYANURIC ACID may vigorously react with small quantities of water releasing chlorine gas. | | Reactivity Profile | DICHLOROISOCYANURIC ACID is slightly hygroscopic and is unstable in the presence of DMSO. This is an oxidizing material; DICHLOROISOCYANURIC ACID may ignite organic compounds with which DICHLOROISOCYANURIC ACID comes in contact. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Agricultural Uses | Biocide, Water treatment chemical: Used in disinfectants and cleaning solution in domestic
products and in food-processing plants. Registered
because there are many products that contain this
chemical. | | Trade name | ACL 70®; CDB 60®; FI CLOR 71®;
HILITE 60®; ORCED®; TROCLOSENE® | | Safety Profile | Moderately toxic by
ingestion. Human systemic effects by
ingestion: ulceration or bleeding from
stomach. Autopsy findings include
gastrointestinal tract irritation, tissue edema,
liver and kidney congestion. A severe eye
and skin irritant. When heated to
decomposition it emits chlorides and carbon
monoxide. | | Potential Exposure | This triazinetrione biocide, and water
treatment chemical used in disinfectants and cleaning
solution in domestic products and in food processing plants | | Shipping | UN2465 Dichloroisocyanuric acid, dry, or
Dichloroisocyanuric acid salts., Hazard Class: 5.1; Labels:
5.1-Oxidizer. It is a marine pollutant and environmentally
hazardous substance. | | Incompatibilities | A strong oxidizer and chlorinating
compound. Violent reaction with organic and flammable
materials. Contact with materials containing nitrogen,
such as ammonia, ammonium salts, or urea, may be violent
and form highly explosive nitrogen trichloride. Contact
with water forms hypochlorous acid and evolves extremely
dense and noxious fumes of chlorides; chlorine gas.
Triazine compounds are incompatible with oxidizers, acids,
acid chlorides, acid halides, isocyanates, halogenated
organics, peroxides, phenols (acidic), epoxides, and
anhydrides. Contact with strong reducing agents such
as hydrides may generate explosive a flammable gas | | Waste Disposal | Recycle any unused portion
of the material for its approved use or return it to the
manufacturer or supplier. Generators of waste containing
this contaminant (≧100 kg/mo) must conform with EPA
regulations governing storage, transportation, treatment,
and waste disposal. Incineration with effluent gas scrubbing
is recommended. Containers must be disposed of properly
by following package label directions or by contacting
your local or federal environmental control agency, or by
contacting your regional EPA office. Ultimate disposal of
the chemical must consider: the material’s impact on air
quality; potential migration in soil or water; effects on
animal, aquatic, and plant life; and conformance with
environmental and public health regulations |
| | DICHLOROISOCYANURIC ACID Preparation Products And Raw materials |
|