|
|
| | RILPIVIRINE HCL Basic information |
| Product Name: | RILPIVIRINE HCL | | Synonyms: | RILPIVIRINE HCL;Rilpivirine-d6 HCl;Rilpivirine HCl (TMC278;TMC 278. trade name Edurant;Rilpivirine Hydrochloride;Ripivirine Hydrochloride | | CAS: | 700361-47-3 | | MF: | C22H19ClN6 | | MW: | 402.89 | | EINECS: | | | Product Categories: | 700361-47-3 | | Mol File: | 700361-47-3.mol | 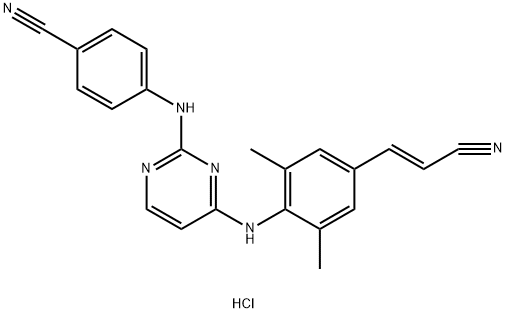 |
| | RILPIVIRINE HCL Chemical Properties |
| storage temp. | Store at -20°C | | solubility | DMSO:50.0(Max Conc. mg/mL);136.46(Max Conc. mM) | | form | Solid | | color | White to off-white |
| | RILPIVIRINE HCL Usage And Synthesis |
| Uses | Rilpivirine Hydrochloride was shown to be able to treat and or prevent immunodeficiency virus-1. It also has uses for anti-viral therapy | | Definition | ChEBI: A hydrochloride obtained by reaction of rilpivirine with one equivalent of hydrochloric acid. Used for treatment of HIV. | | Clinical Use | Rilpivirine hydrochloride (Edurant), a non-nucleoside reverse
transcriptase inhibitor (NNRTI), received its approval both from
the U.S. FDA and E.U. EMA in 2011 for the treatment of HIV-1 infection in treatment-na?ve adult patients. It was discovered and
developed by Janssen Pharmaceuticals and its subsidiary Tibotec
Pharmaceuticals. As a second generation NNRTI, rilpivirine hydrochloride
displayed higher potency and longer half-life with a 25 mg
once a day dose, compared to existing NNRTIs, such as the 200 mg
BID of efavirenze (Sustiva). In late 2011, the fixed-dose
combination products of rilpivirine hydrochloride with two
nucleoside reverse transcriptase inhibitor (RTIs) emtricitabine and tenofovir disoproxil fumarate, co-developed by Gilead Science
and Tibotec, were also approved both by the FDA and EMA under
brand names Complera® and Eviplera®, respectively. | | Synthesis | Similar
to efavirenze, rilpivirine hydrochloride is a diarylpyrimidine
(DAPY) compound, and the large-scale process synthesis begins
with commercially available 2-methylthio-4-pyrimidinone (193)
shown in the scheme. 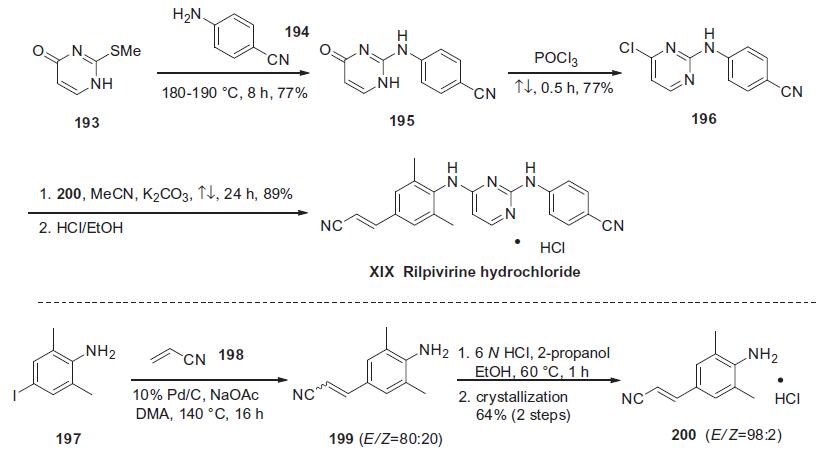
Thioether 193 was condensed with neat 4-cyanoaniline (194) at
elevated temperature to afford diarylamine 195 in 77% yield. Subsequent
treatment of pyrimidone 195 with refluxing POCl3 provided
the corresponding chloride 196 in 77% yield.160,161 In the
presence of K2CO3, chloride 196 was treated with the (E)-cinnamonitrile
aniline 200 to give rilpivirine hydrochloride (XIX) in good
yield.158 Aniline 200 was prepared via a Heck reaction of commercially
available 4-iodo-2,6-dimethyl-benzeneamine (197) and
acrylonitrile (198) affording compound 199 as a 4:1 mixture of E/Z isomers. The distribution of E/Z olefins was increased to 98:2
by salt formation and recrystallization to ultimately provide pure
(E)-200 in 64% yield for two steps. | | in vivo | R278474 (10-160 mg/kg; p.o. for 1 month) does not produce abnormal effects in rat, apart from liver weight increase and species-specific thyroid hypertrophy, both at the higher dose levels[1].
R278474 (i.v.) exhibits elimination half-life ranges from 4.4 h in rat to 31 h in dog, and exposure (AUCinf) amounts to 3.1 μg h/mL (4 mg/kg) in rat, 8.7 μg h/mL (1.25 mg/kg) in dog, 1.4 μg h/mL (1.25 mg/ kg) in monkey, and 44 μg h/mL (1.25 mg/kg) in rabbit[1].
R278474 (p.o.) exhibits half-life ranges between 2.8 h in rat and 39 h in dog, and oral bioavailability of 32% and 31% in rat and dog[1]. |
| | RILPIVIRINE HCL Preparation Products And Raw materials |
|