| Product Name: | Suvorexant | | Synonyms: | 5-Chloro-2-[(5R)-5-methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoyl]-1,4-diazepan-1-yl]-1,3-benzoxazole;MK 4305;Suvorexant;MK-4305 (Suvorexant);Methanone, [(7R)-4-(5-chloro-2-benzoxazolyl)hexahydro-7-Methyl-1H-1,4-diazepin-1-yl][5-Methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]-;Suvorexant (MK-4305);Suvorexant (with 4 ints.);SUVOREXABT | | CAS: | 1030377-33-3 | | MF: | C23H23ClN6O2 | | MW: | 450.92 | | EINECS: | 685-109-7 | | Product Categories: | API;Inhibitors;1030377-33-3 | | Mol File: | 1030377-33-3.mol | 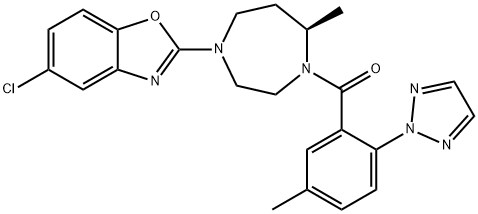 |
| | Suvorexant Chemical Properties |
| Melting point | 153℃ | | Boiling point | 669.8±65.0 °C(Predicted) | | density | 1.41 | | storage temp. | Room Temperature | | solubility | Acetonitrile (Slightly), Chloroform (Slightly), DMSO (Slightly, Heated) | | form | Solid | | pka | 1.47±0.40(Predicted) | | color | White to Pale Beige | | InChIKey | JYTNQNCOQXFQPK-MRXNPFEDSA-N | | SMILES | C(N1[C@H](C)CCN(C2=NC3=CC(Cl)=CC=C3O2)CC1)(C1=CC(C)=CC=C1N1N=CC=N1)=O |
| DEA Controlled Substances | CSCN: 2223
CAS SCH: IV
NARC: N |
| | Suvorexant Usage And Synthesis |
| description | Suvorexant (Belsomra®) is a novel sedative hypnotic drug that is prescribed to promote sleep in patients with insomnia. It is the first of a new class of drugs classified as dual orexin receptor antagonists (DORAs) and is currently approved for the treatment of insomnia in the United States and Japan. It is a CNS depressant and blocks the binding of wake-promoting neuropeptides orexin A and orexin B to the two orexin receptors (OX1R and OX2R) thus, altering the signaling (action) of orexin in the brain and suppressing the sleep-wake drive.
Suvorexant is a central nervous system (CNS) depressant and can impair daytime wakefulness even when used as prescribed. Prescribers should monitor for somnolence and CNS depressant effects, but impairment can occur in the absence of symptoms, and may not be reliably detected by ordinary clinical exam. CNS depressant effects may persist in some patients for up to several days after discontinuing Suvorexant.
Belsomra (Suvorexant) can impair driving skills and may increase the risk of falling asleep while driving. Discontinue or decrease the dose in patients who drive if daytime somnolence develops. Caution patients taking Belsomra 20 mg against next-day driving and other activities requiring full mental alertness. Caution patients taking lower doses of Belsomra as well, because there is individual variation in sensitivity to Belsomra. | | Pharmacokinetics | In fasting conditions, the median time to maximum plasma concentration of suvorexant is 2 h. For suvorexant 10 mg, the mean absolute bioavailability is 82 %. Administration of suvorexant with a high-fat meal had no clinically significant on systemic drug exposure, but delayed tmax by 1.5 h. Steady state is achieved within 3 days. The mean volume of distribution of suvorexant is 49 L. Suvorexant is extensively bound to plasma proteins, including albumin and a1-acid glycoprotein, and does not preferentially distribute into the red blood cells.
Suvorexant is primarily metabolized by CYP3A, with a minor contribution by CYP2C19. The main circulating compounds are the unchanged drug and a hydroxyl metabolite, which is not expected to be pharmacologically active. Suvorexant is primarily eliminated in the faeces. | | Mechanism of action | Suvorexant is a dual antagonist of orexin receptors OX1R and OX2R. It exerts its pharmacological effect by inhibiting binding of neuropeptides orexin A and B, also known as hypocretin 1 and 2, that are produced by neurons in the lateral hypothalamus. These neurons control the wake-promoting centers of the brain and are active during wakefulness, especially during motor activities, and stop firing during sleep. By inhibiting the reinforcement of arousal systems, suvorexant use causes a decrease in arousal and wakefulness, rather than having a direct sleep-promoting effect.
www.drugbank.ca/drugs/DB09034 | | side effects | Suvorexant (BELSOMRA) has possible side effects. Before you begin treatment, it’s important to know what these side effects are and to discuss the risks and benefits of taking BELSOMRA with your doctor.
Below is a list of the most commonly reported adverse events that occurred in 3-month clinical trials:
placebo (n=767) BELSOMRA 15 mg or 20 mg (n=493)
- Diarrhea (placebo 1%, BELSOMRA 2%)
- Dry mouth (placebo 1%, BELSOMRA 2%)
- Upper respiratory tract infection (placebo 1%, BELSOMRA 2%)
- Headache (placebo 6%, BELSOMRA 7%)
- Next-day drowsiness (placebo 3%, BELSOMRA 7%)
- Dizziness (placebo 2%, BELSOMRA 3%)
- Abnormal dreams (placebo 1%, BELSOMRA 2%)
- Cough (placebo 1%, BELSOMRA 2%)
Side effects that you should report to your doctor or health care professional as soon as possible:
- allergic reactions like skin rash, itching or hives, swelling of the face, lips, or tongue
- confusion
- depressed mood
- feeling faint or lightheaded, falls
- hallucinations
- inability to move or speak for up to several minutes while you are going to sleep or waking up
- memory loss
- periods of leg weakness lasting from seconds to a few minutes
- problems with balance, speaking, walking
- restlessness, excitability, or feelings of agitation
- unusual activities while asleep like driving, eating, making phone calls
Side effects that usually do not require medical attention (Report these to your doctor or health care professional if they continue or are bothersome.):
- abnormal dreams
- daytime drowsiness
- diarrhea
- dizziness
- headache
| | Dosage | The recommended dosage is 10 mg, to be taken orally no more than once per night, within 30 min of going to bed and with at least 7 h remaining until the planned awaken time. If this dosage is well tolerated but not effective, the dosage can be increased up to a maximum of 20 mg once daily; the lowest effective dosage should be used. Patients taking concomitant moderate cytochrome P450 (CYP) 3A4 inhibitors should receive a reduced dose of 5 mg.
| | Description | Suvorexant, a dual orexin receptor antagonist marketed under
the trade name Belsomra®, discovered and developed by Merck
for the treatment of insomnia, was approved by the US FDA in
August 2014 and became available in Japan in November of the
same year. The drug’s mechanism of action operates through
the competitive blockade of wake-promoting neuropeptides orexin
A and orexin B toward receptors orexin receptor type 1 and orexin
receptor type 2, which are believed to modulate sleep-wake
cycles. | | Uses | Suvorexant (MK-4305) is a dual (non-selective) orexin receptor antagonist in development by Merck & Co. for the treatment of insomnia. It works by turning off wakefulness rather than by inducing sleep. Users of higher doses had an increased rate of suicidal ideation. | | Definition | ChEBI: Suvorexant is an aromatic amide obtained by formal condensation of the carboxy group of 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid with the secondary amino group of 5-chloro-2-[(5R)-5-methyl-1,4-diazepan-1-yl]-1,3-benzoxazole. An orexin receptor antagonist used for the management of insomnia. It has a role as a central nervous system depressant and an orexin receptor antagonist. It is a member of 1,3-benzoxazoles, a member of triazoles, a diazepine, an aromatic amide and an organochlorine compound. | | Biological Activity | Suvorexant is a dual orexin receptor (OXR) antagonist that blocks both OX1R and OX2R (Kis = 1.2 and 0.60 nM, respectively). It reduces locomotor activity and promotes sleep by inhibiting the binding of orexin A and B. In rats, suvorexant decreases self-administration of, and conditioned place preference for, cocaine ( | 16186 | ISO60176). It also decreases dopamine levels in the rat ventral striatum following a cocaine-induced increase. Formulations containing suvorexant are used in the treatment of insomnia. Suvorexant is regulated as a Schedule IV compound in the United States. This compound is also available as an analytical reference standard. | | Synthesis | Commercially available acid 240 was first
subjected to a copper-assisted substitution reaction involving
1,2,3-triazole in DMF at elevated temperatures. Although these
conditions resulted in an excellent yield of a triazole-substituted
product, an approximate 4:1 ratio of the desired 2-arylated triazole
241 and the undesired 1-arylated triazole byproduct were recovered
from the reaction. The mixture was then treated with N,Ndimethylethylenediamine
in acid to sequester copper. Next, the
mixture of arylated triazoles was carefully subjected to sodium tbutoxide
in DMF and ethyl acetate to form the corresponding
sodium salts, and interestingly it was found that the desired
sodium salt of 241 could be isolated based on its solubility profile
under these conditions. Acidification of the desired carboxylate salt
using dilute HCl gave rise to acid 241 in 60% yield across the fourstep
sequence. Next, subjection of this acid to oxalyl chloride in
chilled DMF generated the acid chloride 242 in excellent yield. This
crude acid chloride was used immediately in the next step of the
synthetic sequence.
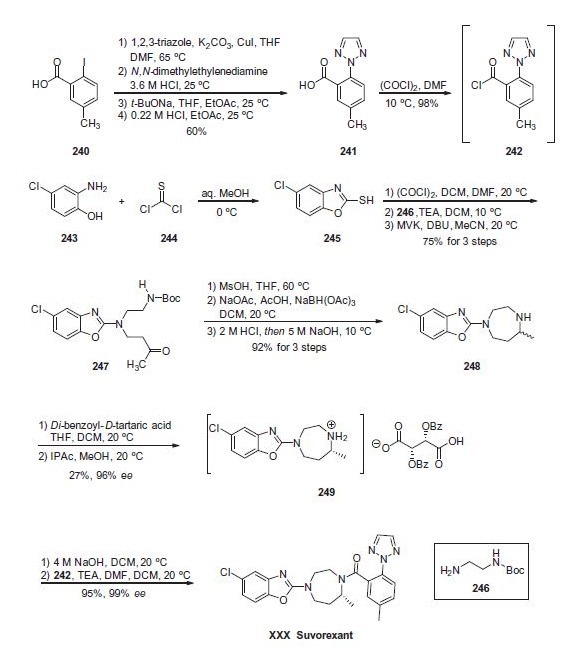
For the preparation of the diazepine-containing portion of
suvorexant, the synthesis commenced with the condensation of
commercial 2-amino-4-chlorophenol (243) with thiophosgene
(244) to furnish benzoxazole 245. Next, thiol 245
was converted to the corresponding chloride prior to exposure to
Boc-protected ethylenediamine 246 under basic conditions. This
was followed by a Michael addition of the resulting aminobenzoxazole
and methyl vinyl ketone (MVK). The result of this sequence of
reactions delivered aminobenzoxazole ketone 247 in 75% yield
over the three steps. Next, subjection of the carbamate to methanesulfonic acid removed the Boc functionality and this was
followed by an intramolecular reductive amination sequence to
construct the diazaepine ring. Acid¨Cbase workup ultimately provided
the racemic diazepine 248 in 92% yield from 247. Next, salt
formation with a benzoyl tartaric acid and subsequent recrystallization
upgrade using isopropyl acetate and methanol at ambient
temperature was used to resolve racemic 248 into the tartrate salt
249 in 27% yield and excellent enantiomeric excess. Finally, salt
249 was freebased using sodium hydroxide prior to exposure to
the crude acid chloride 242 under basic conditions to ultimately
deliver suvorexant (XXX) in 95% yield and 99% ee across the twostep
sequence. | | target | OX1 receptor | | Metabolism | Suvorexant is primarily metabolized by cytochrome-P450 3A4 enzyme (CYP3A4) with a minor contribution from CYP2C19. Major circulating metabolites are suvorexant and a hydroxy-suvorexant metabolite, which is not expected to be pharmacologically active. There is potential for drug-drug interactions with drugs that inhibit or induce CYP3A4 activity. |
| | Suvorexant Preparation Products And Raw materials |
|