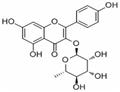
- Afzelin
-
- $0.00 / 5mg
-
2023-02-24
- CAS:482-39-3
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
|
| | Afzelin Basic information |
| Product Name: | Afzelin | | Synonyms: | KAEMPFEROL 3-O-GLUCORHAMNOSIDE;2-(4-Hydroxyphenyl)-4-oxo-5,7-dihydroxy-4H-1-benzopyran-3-yl α-L-rhamnopyranoside;3-(α-L-Rhamnopyranosyloxy)-5,7,4'-trihydroxyflavone;3-[(6-Deoxy-α-L-mannopyranosyl)oxy]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one;KAEMPFERIN; KAEMPFEROL-3-RHAMNOSIDE;4H-1-Benzopyran-4-one,3-[(6-deoxy-a-L-Mannopyranosyl)oxy]-5,7-dihydroxy-2-(4-hydroxyphenyl)-;Afzelin/Kaempferol 3-o-glucorhamnoside;Kaempferol 3-O-α-L-Rhamnoside | | CAS: | 482-39-3 | | MF: | C21H20O10 | | MW: | 432.38 | | EINECS: | | | Product Categories: | | | Mol File: | 482-39-3.mol | 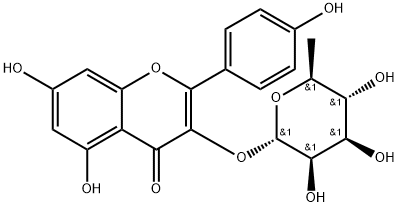 |
| | Afzelin Chemical Properties |
| Melting point | 172-174 °C (decomp)(Solv: water (7732-18-5)) | | Boiling point | 765.6±60.0 °C(Predicted) | | density | 1.70±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | Soluble in methan | | form | powder | | pka | 6.20±0.40(Predicted) | | color | Yellow |
| | Afzelin Usage And Synthesis |
| Description | Afzelin (Synonyms: Kaempferol-3-O-rhamnoside) is a flavonoid isolated from Thesium chinense Turcz. and widely distributed in Korea and China. It has anti-inflammatory, anti-oxidative stress response, anti-apoptotic, and anti-cardiac cytotoxic effects. AfzelinIt can reduce mitochondrial damage, enhance mitochondrial biosynthesis, and reduce mitochondria-related proteins. Parkinand PTENinduced putative kinase 1 (putative kinase 1)s level. AfzelinCan be improved D-galactosamine(GalN)/LPSSurvival rate of mice treated with doxorubicin prophylaxis Induced cardiotoxicity and scopolamine -induced neurological injury. AfzelinAlso inhibits asthma and allergies caused by ovalbumin. | | Uses | Kaempferol 3-O-α-L-Rhamnoside is found in Cornus macrophylla and has shown to have antibacterial activity against Pseudomonas aeruginosa, a leading cause of illness in immunocompromised individuals. | | Definition | ChEBI: Afzelin is a glycosyloxyflavone that is kaempferol attached to an alpha-L-rhamnosyl residue at position 3 via a glycosidic linkage. It has a role as a plant metabolite, an antibacterial agent and an anti-inflammatory agent. It is a glycosyloxyflavone, a trihydroxyflavone and a monosaccharide derivative. It is functionally related to a kaempferol. It is a conjugate acid of an afzelin(1-). | | References | [1] SO-YOUNG OH. Central administration of afzelin extracted from Ribes fasciculatum improves cognitive and memory function in a mouse model of dementia.[J]. Scientific Reports, 2021: 9182. DOI:10.1038/s41598-021-88463-6.
[2] LEI XIA. Afzelin induces immunogenic cell death against lung cancer by targeting NQO2.[J]. BMC Complementary Medicine and Therapies, 2023, 23 1: 381. DOI:10.1186/s12906-023-04221-3.
[3] EVA RACHMI. Identification of afzelin potential targets in inhibiting triple-negative breast cancer cell migration using reverse docking.[J]. Porto biomedical journal, 2020, 5 6: e095. DOI:10.1097/j.pbj.0000000000000095.
[4] SIMPLICE JOEL NDENDOUNG TATSIMO. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum.[J]. BMC Research Notes, 2012, 5: 158. DOI:10.1186/1756-0500-5-158.
[5] YOUNG-KYOON KIM. Isolation of flavonol rhamnosides fromloranthus tanakae and cytotoxic effect of them on human tumor cell lines[J]. Archives of Pharmacal Research, 2004, 27 1: 44-47. DOI:10.1007/BF02980044.
[6] VINIT RAJ. Antiviral activities of 4H-chromen-4-one scaffold-containing flavonoids against SARS–CoV–2 using computational and in vitro approaches[J]. Journal of Molecular Liquids, 2022, 353: Article 118775. DOI:10.1016/j.molliq.2022.118775.
[7] BORBáLA VERMES . The synthesis of afzelin, paeonoside and kaempferol 3-O-β-rutinoside[J]. Phytochemistry, 1976, 15 8: Pages 1320-1321. DOI:10.1016/0031-9422(76)85106-0. |
| | Afzelin Preparation Products And Raw materials |
|