|
|
| | 6-BROMO-1H-INDAZOLE-3-CARBALDEHYDE Basic information |
| Product Name: | 6-BROMO-1H-INDAZOLE-3-CARBALDEHYDE | | Synonyms: | Bromoindazolecarboxaldehyde;1H-Indazole-3-carboxaldehyde,6-broMo-;6-BROMO-1H-INDAZOLE-3-CARBALDEHYDE;6-BROMO-1H-INDAZOLE-3-CARBOXALDEHYDE;6-BROMO-INDAZOLE-3-CARBOXALDEHYDE;1-(6-bromo-1H-indazol-3-yl)-N,N-dimethylmethanamine;6-Bromo-1H-indazole-3-carbaldehyde 97%;6-Bromo-1H-indazole-3-carboxyaldehyde | | CAS: | 885271-72-7 | | MF: | C8H5BrN2O | | MW: | 225.04 | | EINECS: | | | Product Categories: | Indazole | | Mol File: | 885271-72-7.mol | 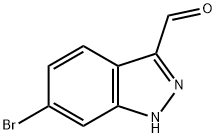 |
| | 6-BROMO-1H-INDAZOLE-3-CARBALDEHYDE Chemical Properties |
| Boiling point | 414.1±25.0 °C(Predicted) | | density | 1.830±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | pka | 10.82±0.40(Predicted) | | form | solid | | color | Brown |
| | 6-BROMO-1H-INDAZOLE-3-CARBALDEHYDE Usage And Synthesis |
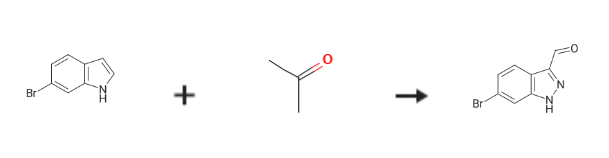
| Uses | 6-Bromo-1H-indazole-3-carbaldehyde is an indazole compound, mostly used in chemical manufacturing or pharmaceutical intermediate components. | | Synthesis | 6-Bromo-1H-indazole-3-carbaldehyde is prepared by the reaction of 6-Bromoindole and acetone. The specific synthesis steps are as follows:
To 70g is added in an aqueous solution of sodium nitrite of 20g 6-bromoindole acetone: water (200 ml: 200 ml) solution; room temperature, in to the system by adding 2N HCl solution to pH to 2.5 the left and right; 20 min the rear, with a red brown gas desorbing, slabs system has; 10 min later, TLC detection material disappears, cessation of the reaction, adding to system 1.0L ethyl acetate (EA), two-phase separation, the organic phase with saturated sodium bicarbonate (500 ml * 2) washing, drying organic anhydrous magnesium sulfate, concentrated to obtain 22g compound 2, as a black solid.
 |
| | 6-BROMO-1H-INDAZOLE-3-CARBALDEHYDE Preparation Products And Raw materials |
|