- 2-Hexylcyclopentanone
-

- $0.00 / 100g
-
2020-02-26
- CAS: 13074-65-2
- Min. Order: 100g
- Purity: 99.0%+
- Supply Ability: 100 tons
- 2-N-HEXYLCYCLOPENTANONE
-

- $1.00 / 1KG
-
2020-02-02
- CAS:13074-65-2
- Min. Order: 1KG
- Purity: 99%HPLC
- Supply Ability: 100KG
|
| | 2-N-HEXYLCYCLOPENTANONE Basic information |
| Product Name: | 2-N-HEXYLCYCLOPENTANONE | | Synonyms: | 2-hexyl-cyclopentanon;hexylcyclopentanone;JASMATONE;2-N-HEXYLCYCLOPENTANONE;2-HEXYLCYCLOPENTANONE;2-Hexyl-1-cyclopentanone;2-hexylcyclopentan-1-one;Cyclopentanone, 2-hexyl- | | CAS: | 13074-65-2 | | MF: | C11H20O | | MW: | 168.28 | | EINECS: | 235-970-6 | | Product Categories: | | | Mol File: | 13074-65-2.mol | 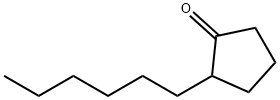 |
| | 2-N-HEXYLCYCLOPENTANONE Chemical Properties |
| RTECS | GY4972000 | | TSCA | Yes | | HS Code | 2914290090 |
| Provider | Language |
|
ALFA
| English |
| | 2-N-HEXYLCYCLOPENTANONE Usage And Synthesis |
| Description | 2-N-hexylcyclopentanone is less spicy or Celery-like than Jasmone, more sharp-fruity or green, and not quite as powerful. Rather than considering it as a lowcost substitute for Jasmone, it would be better to use it as an individual fragrance material with effects of its own. It will lend considerable floral-herbaceous power to fragrances other than the typical floral ones, and itmay participate in giving Jasmin effect when blended with suitable components. | | Preparation | 2-N-hexylcyclopentanone is prepared:
1) by condensation of Hexaldehyde and CycIopentanone,
followed by hydrogenation
of the condensation product.
2) by hydrogenation of the reaction mixture
from Lactonization of Undecylenic acid
with Phosphoric or Polyphosphoric acid. | | Synthesis Reference(s) | Journal of the American Chemical Society, 98, p. 248, 1976 DOI: 10.1021/ja00417a048 |
| | 2-N-HEXYLCYCLOPENTANONE Preparation Products And Raw materials |
|