| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Bufuralol
CAS:54340-62-4
Purity:98.26% Package:2mg/RMB 3188
|
- Bufuralol
-
- $455.00 / 2mg
-
2024-10-28
- CAS:54340-62-4
- Min. Order:
- Purity: 98.26%
- Supply Ability: 10g
|
| | (+/-)-BUFURALOL HYDROCHLORIDE Basic information |
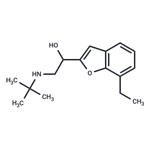
| Product Name: | (+/-)-BUFURALOL HYDROCHLORIDE | | Synonyms: | Bufuralol;BUFARALOL;2-[1-Hydroxy-2-(tert-butylamino)ethyl]-7-ethylbenzofuran;2-[2-(t-Butylamino)-1-hydroxyethyl]-7-ethylbenzofuran;Bufural;α-[(tert-Butylamino)methyl]-7-ethyl-2-benzofuranmethanol;(Rs)-alpha-(tert-butylamino)methyl)-7-ethyl-12-benzofuranmethanol;1-(7-Ethylbenzofuran-2-yl)-2-tert-butylamino-1-hydroxyethane | | CAS: | 54340-62-4 | | MF: | C16H23NO2 | | MW: | 261.363 | | EINECS: | 2591125 | | Product Categories: | | | Mol File: | 54340-62-4.mol |  |
| | (+/-)-BUFURALOL HYDROCHLORIDE Chemical Properties |
| Melting point | 143-146 °C | | Boiling point | 393.2±37.0 °C(Predicted) | | density | 1.066±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | form | Solid | | pka | pKa 8.97 (Uncertain) | | color | white to off-white |
| Hazard Codes | Xn | | Risk Statements | 22 | | WGK Germany | 3 |
| | (+/-)-BUFURALOL HYDROCHLORIDE Usage And Synthesis |
| Originator | Bururalol
hydrochloride,Onbio Inc. | | Definition | ChEBI: Bufuralol is a member of benzofurans. | | Manufacturing Process | 68.3 g (0.182 mol) of trimethyl-phenyl-ammonium perbromide were added in
a single portion at 20°C to a stirred solution of 48.5 g (0.182 mol) of 5-
bromo-2-acetyl-7-ethylbenzofuran in 400 ml of dry tetrahydro-furan. The resulting mixture was stirred at 20°C for 3 h, during which time trimethylphenyl-
ammonium bromide precipitated out. The mixture was then poured
into water and extracted 3 times with ether. The combined ether extracts were
washed successively with water, saturated sodium bicarbonate solution, water
and saturated brine, dried over anhydrous sodium sulfate, filtered and
evaporated under reduced pressure. The solid residue was recrystallized from
ethanol to yield 43.1 g of 5-bromo-2-bromoacetyl-7-ethylbenzofuran as yellow
crystals, melting point 101-102°C.
1.35 g of sodium borohydride were added portion-wise at room temperature
over a period of 20 min to a stirred solution of 17.3 g (0.05 mol) of 5-bromo-
2-bromoacetyl-7-ethylbenzofuran in 100 ml of dioxane and 25 ml of water.
The mixture was stirred at room temperature for 3 h, then dioxane was
removed by evaporation at 40°C under reduced pressure and the residue was
diluted with water and extracted 3 times with ether. The combined ether
extracts were worked up in the usual manner to yield 16.0 g of crude 5-
bromo-2-(2-bromo-1-hydroxyethyl)-7-ethyl-benzofuran as a viscous oil.
16.0 g of crude 5-bromo-2-(2-bromo-1-hydroxyethyl)-7-ethylbenzofuran and
37.0 g of t-butylamine were heated at 100°C in a sealed autoclave for 24 h.
After cooling, excess t-butylamine was evaporated off and the residue was
taken up in dilute aqueous hydrochloric acid. The aqueous solution was
washed twice with ether, basified with dilute aqueous sodium hydroxide
solution and extracted twice with ether. The combined ether extracts were
washed with water and with brine, dried over anhydrous sodium sulfate,
filtered and evaporated. The solid residue was crystallized from petroleum
ether (boiling point 60-80°C) to yield 4.7 g of 5-bromo-2-(2-t-butylamino-1-
hydroxyethyl)-7-ethylbenzofuran as buff crystals, melting point 101-103°C.
4.8 g of 5-bromo-2-(2-t-butylamino-1-hydroxyethyl)-7-ethylbenzofuran in 50
ml of ethanol were hydrogenated at room temperature and atmospheric
pressure in the presence of 0.3 g of 5% palladium-on-carbon catalyst. After
the uptake of one equivalent of hydrogen, the hydrogenation was terminated,
catalyst was filtered off and the filtrate was evaporated to dryness. The
residue was basified and extracted twice with ether. The combined ether
extracts were worked up in the usual manner to give 2-(2-t-butylamino-1-
hydroxyethyl)-7-ethylbenzofuran in the form of an oil.
In practice it is usually used as hydrochloride. | | Therapeutic Function | Beta-adrenergic blocker |
| | (+/-)-BUFURALOL HYDROCHLORIDE Preparation Products And Raw materials |
|