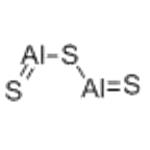
- Aluminium sulfide
-
- $0.00 / 1KG
-
2024-08-17
- CAS:1302-81-4
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10000KG
- ALUMINUM SULFIDE
-

- $15.00 / 1KG
-
2021-07-02
- CAS:1302-81-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Aluminum sulfide
-

- $0.00 / 5g
-
2019-11-18
- CAS:1302-81-4
- Min. Order: 5g
- Purity: 99.9%
- Supply Ability: 10kg
Related articles - What is the name for Al2S3?
- Aluminum Sulfide has a chemical formula of Al2S3, it may be of value for its photovoltaic properties at smaller scales.
- Feb 27,2024
|
| | Aluminium sulfide Basic information |
| | Aluminium sulfide Chemical Properties |
| Melting point | 1100 °C | | density | 2.02 g/mL at 25 °C (lit.) | | form | granular | | Specific Gravity | 2.02 | | color | Yellow | | Water Solubility | decomposes in H2O [MER06] | | Sensitive | Moisture Sensitive | | Crystal Structure | Hexagonal, Wurtzite (Zincite) Structure - Space Group P 63mc | | crystal system | Six sides | | Merck | 14,367 | | Solubility Product Constant (Ksp) | pKsp: 6.7 | | Space group | P61 | | Lattice constant | | a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.643 | 0.643 | 1.788 | 90 | 90 | 120 | 0.6402 |
| | CAS DataBase Reference | 1302-81-4(CAS DataBase Reference) | | EPA Substance Registry System | Aluminum sulfide (Al2S3) (1302-81-4) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-27-36/37/39 | | RIDADR | UN3134 | | WGK Germany | 3 | | TSCA | Yes | | HazardClass | 4.3 | | PackingGroup | III |
| | Aluminium sulfide Usage And Synthesis |
| Chemical Properties |
Aluminium sulfide is a greyish solid, with a bad sulfide smell, especially in moist air. It has the chemical formula Al2S3. It reacts with water to release hydrogen sulfide, and is insoluble in most solvents. It melts at 1100 °C and sublimes at 1,500 °C. Aluminium sulfide has at least six crystalline forms, the more important ones being α, β, γ and δ.
| | Uses | Aluminum Sulfide is used in the preparation of hydrogen sulfide.
Used to produce chemicals used in the tanning and papermaking industry.
Used to produce organic compounds such as ethanethiol. | | Uses | To prepare hydrogen sulfide. | | Preparation | Aluminium sulfide can be prepared through a thermite-like reaction between aluminium and sulfur powders.
2 Al + 3 S → Al2S3 | | Hazard | Irritant to skin and mucous membranes. |
| | Aluminium sulfide Preparation Products And Raw materials |
|