|
|
| | Methyl 1,2,4-triazole-3-carboxylate Basic information | | Uses |
| Product Name: | Methyl 1,2,4-triazole-3-carboxylate | | Synonyms: | METHYL 1,2,4-TRIAZOLE-3-CARBOXYLATE;1H-TRIAZOLE-3-CARBOXYLIC ACID METHYL ESTER;1H-1,2,4-TRIAZOLE-3-CARBOXYLIC ACID METHYL ESTER;1,2,4-Triatoze-3-carboxylic acid Methylester;1,2,4-Triazole-3-methyl carboxylate;1.2.4-triazole-3-methyl-carboxylata;1H-1,2,4-Triazole-3-methylcarboxylate;1,2,4-Triazole-3-CarboxylataAcidMethylEster | | CAS: | 4928-88-5 | | MF: | C4H5N3O2 | | MW: | 127.1 | | EINECS: | 480-470-5 | | Product Categories: | Thiophenes;Aromatic Esters;Building Blocks;Heterocyclic Building Blocks;Triazoles;carboxylic ester;1;Intermediates | | Mol File: | 4928-88-5.mol | 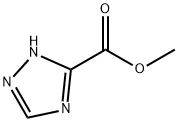 |
| | Methyl 1,2,4-triazole-3-carboxylate Chemical Properties |
| Melting point | 196-199 °C(lit.) | | Boiling point | 283.9±23.0 °C(Predicted) | | density | 1.380±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Liquid | | pka | 7.96±0.20(Predicted) | | color | Clear colorless to yellow | | InChI | InChI=1S/C4H5N3O2/c1-9-4(8)3-5-2-6-7-3/h2H,1H3,(H,5,6,7) | | InChIKey | QMPFMODFBNEYJH-UHFFFAOYSA-N | | SMILES | N1C(C(OC)=O)=NC=N1 | | CAS DataBase Reference | 4928-88-5(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36-37 | | WGK Germany | 3 | | HS Code | 29339900 |
| | Methyl 1,2,4-triazole-3-carboxylate Usage And Synthesis |
| Uses | Methyl 1,2,4-Triazole-3-carboxylate has been used as a reactant for the preparation of Ribavirin (1-β-D-ribofuranosyl)-1,2,4-triazole-3-carboxamide, an antiviral agent. | | Chemical Properties | White solid | | Uses | Methyl-1H-1,2,4-triazole-3-carboxylate (1,2,4-Triazole-3-methylcarboxylate) may be used in the preparation of 1H-1,2,4-triazole-3-carbohydrazide.
It may also be used in the synthesis of the following nucleoside analogues:
- 1-(2,2-dimethyl-6-trityloxymethyl-4,6a-dihydro-3aH-cyclopenta-[1,3]dioxol-4-yl)-1H-1,2,4-triazole-3-carboxylic acid methyl ester
- methyl 4,6-O-benzylidene-2-(methyl 1H-1,2,4-triazol-1-yl-3-carboxylate)-2-deoxy-<SC>D</SC>-altrohexopyranoside
- 1,5-anhydro-4,6-O-benzylidene-2-(methyl 1H-1,2,4-triazol-1-yl-3-carboxylate)-2-deoxy-<SC>D</SC>-altro-hexitol
| | Uses | Methyl 1,2,4-Triazole-3-carboxylate has been used as a reactant for the preparation of Ribavirin (1-β-D-ribofuranosyl)-1,2,4-triazole-3-carboxamide, an antiviral agent. | | General Description | Methyl-1H-1,2,4-triazole-3-carboxylate can be synthesized from 5-amino-1,2,4-triazole-3-carboxylic acid via esterification with methanol. It is utilized as precursor for preparing the nucleoside analogue, Ribavirin. The crystal structure of methyl-1H-1,2,4-triazole-3-carboxylate has been analyzed. | | Synthesis | The product Methyl 1,2,4-triazole-3-carboxylate is synthesized using lime nitrogen as the raw material, and hydrazinolysis, condensation, esterification, deamination and other steps are performed to obtain the product Methyl 1,2,4-triazole-3-carboxylate. The synthesis route using lime nitrogen as the starting material: lime nitrogen first reacts with hydrazine hydrate to obtain a nitrile addition product, and then condenses with oxalic acid to obtain 1,2,4-triazole-3-carboxylic acid methyl ester-5-amino. The deamination process generally requires diazotization and then nitrogen release in an alcohol solution to obtain the product Methyl 1,2,4-triazole-3-carboxylate in which the amino group is replaced by hydrogen.
Synthesis of ethyl cyanoformate: Using ethyl cyanoformate as the raw material, it is subjected to addition with formic acid hydrazide and then cyclization to obtain ethyl 1,2,4-triazole-3-carboxylate, and then alcoholysis with methanol to obtain methyl 1,2,4-triazole-3-carboxylate. Ethyl cyanoformate is used as a raw material and reacted with ethanol to obtain ethoxyamidine hydrochloride, which is then reacted with formic hydrazide for addition reaction, and then cyclized under high temperature conditions to obtain 1,2,4-triazole-3-carboxylic acid ethyl ester, and then ester exchange with methanol is carried out to obtain the final product. |
| | Methyl 1,2,4-triazole-3-carboxylate Preparation Products And Raw materials |
|