| Company Name: |
LinkChem Co.,Ltd.
|
| Tel: |
021-021-58950110 13124863828 |
| Email: |
sales@linkchem.cn |
| Products Intro: |
Product Name:3,3′-diamino-4,4′-dihydroxydiphenyl ether
CAS:6423-17-2
Purity:95%+
|
Phenol, 4,4'-oxybis[2-amino- manufacturers
|
| | Phenol, 4,4'-oxybis[2-amino- Basic information |
| Product Name: | Phenol, 4,4'-oxybis[2-amino- | | Synonyms: | Phenol, 4,4'-oxybis[2-amino-;3,3′-diamino-4,4′-dihydroxydiphenyl ether;3,3'-DIAMINO-4,4'-DIHYDROXYBIPHENYL ETHER;4,4'-oxybis(2-aminophenol);Phenol, 4,4'-oxybis[2-amino- | | CAS: | 6423-17-2 | | MF: | C12H12N2O3 | | MW: | 232.24 | | EINECS: | | | Product Categories: | | | Mol File: | 6423-17-2.mol | 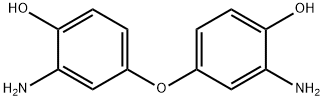 |
| | Phenol, 4,4'-oxybis[2-amino- Chemical Properties |
| Boiling point | 441.4±45.0 °C(Predicted) | | density | 1.438±0.06 g/cm3(Predicted) | | pka | 9.50±0.20(Predicted) |
| | Phenol, 4,4'-oxybis[2-amino- Usage And Synthesis |
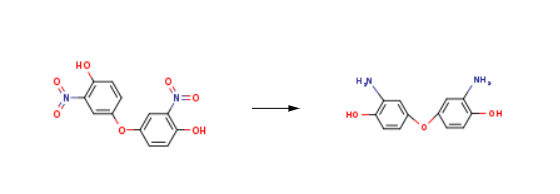
| Synthesis | Phenol, 4,4'-oxybis[2-amino- is synthesised using 3,3'-dinitro-4,4'-dihydroxydiphenyl ether as a raw material by chemical reaction. The specific synthesis steps are as follows:
Di-hydroxy-di-phenyl ether (50.25 gm, 0.28 mol) was dinitrated at the 3,3' positions by suspending in 342 ml of water with cooling, while treating slowly with 265 gm of concentrated nitric acid. After all of the nitric acid was added, the solution became homogeneous. The reaction was exothermic and was kept at less than 25 °C while the nitration product precipitates over about 5 hours. The crystals were collected by filtration and dissolved in minimal hot water, and the solution was adjusted to pH = 6 with aqueous ammonia. Crystals of the dinitro product were immediately precipitated for isolation by filtration. A further crystallization from water with carbon decolorization was done to provide a product suited to reduction: 146 gm (0.5 mol) of this product was dissolved in 1.5 L methanol and reduced under pressure with 8.0 gm of Paladium/Charcoal. When completed, the filtrate was evaporated in vacuo at less than 30 °C. The residue was purified from hot ethanol to yield about 43 grams of product. This product (40 grams) was dissolved in 80 ml acetic acid and added slowly to 40 ml concentrated sulfuric acid with stirring, keeping the temperature below 20 °C. This solution was added carefully over several hours at about 0 °C to a solution containing 125 ml concentrated sulfuric acid and 17.5 gm of sodium nitrite. The reaction was continued for another hour at 0 °C. A second solution was prepared by adding sodium iodide (80 gm), iodine (67 gm), urea (10 gm) in 1300 ml water, and covered with 250 ml of chloroform and the amine solution was added slowly. The temperature rose as the reaction continued. After about an hour, the chloroform layer was saved, and the aqueous layer was extracted 2 more times with 200 ml of chloroform. The combined organic portion was extracted with water and the dried under vacuum in a 40 C water bath. The final product was purified from hot ethanol. Yield was 58 grams.
 |
| | Phenol, 4,4'-oxybis[2-amino- Preparation Products And Raw materials |
|