- METHYL MERCAPTAN
-

- $15.00 / 1KG
-
2021-07-13
- CAS:74-93-1
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- METHYL MERCAPTAN
-

- $15.00 / 1KG
-
2021-07-09
- CAS:74-93-1
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- METHYL MERCAPTAN
-
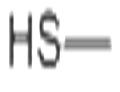
- $100.00 / 25KG
-
2019-07-06
- CAS:74-93-1
- Min. Order: 25KG
- Purity: 98%
- Supply Ability: 1000KG
Related articles - Uses of Methanethiol
- Methanethiol is mainly used to prepare methionine, lysine and tryptophan. Methanethiol is also used as raw materials in the ma....
- Oct 21,2019
|
| | METHYL MERCAPTAN Basic information |
| Product Name: | METHYL MERCAPTAN | | Synonyms: | Methanethiole;methanethiolvialwith25ml;Methvtiolo;Methyl thioalcohol;Methylmercaptaan;methylthioalcohol;Metilmercaptano;Rcra waste number U153 | | CAS: | 74-93-1 | | MF: | CH4S | | MW: | 48.11 | | EINECS: | 200-822-1 | | Product Categories: | API intermediates | | Mol File: | 74-93-1.mol | 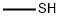 |
| | METHYL MERCAPTAN Chemical Properties |
| Melting point | −123 °C(lit.) | | Boiling point | 6 °C(lit.) | | density | 0.8665 | | vapor density | 1.66 (vs air) | | vapor pressure | 1536 mm Hg ( 20 °C) | | FEMA | 2716 | METHYL MERCAPTAN | | refractive index | 1.4020 (estimate) | | Fp | <71℃ | | solubility | Soluble in alcohol, ether (Weast, 1986), and petroleum naphtha (Hawley, 1981) | | form | liquid | | pka | 10.3(at 25℃) | | Odor | at 0.01 % in propylene glycol. decomposing cabbage garlic | | Odor Type | sulfurous | | explosive limit | 21.8% | | Odor Threshold | 0.00007ppm | | Water Solubility | 23.30 g/L at 20 °C (quoted, Windholz et al., 1983)
0.330 mol/L at 25 °C (Hine and Weimar, 1965) | | JECFA Number | 508 | | Merck | 13,5983 | | BRN | 1696840 | | Henry's Law Constant | 3.03 (Hine and Weimar, 1965) | | Exposure limits | TLV-TWA 0.5 ppm (~1.0 mg/m3 ) (ACGIH
and MSHA); ceiling 10 ppm (OSHA); IDLH
400 ppm (NIOSH); the revised IDLH is 150
ppm in analogy to H2S. | | Stability: | Stable. Highly flammable - note low flash point. Reacts vigorously or explosively with a wide variety of materials - consult a full MSDS data sheet before using. Incompatible with strong oxidizing agents, alkali and alkaline earth metals, epoxides, hydrazines, ketones, lead, mercury (II) oxide, azo- and diazo- compounds, copp | | LogP | 0.72 | | CAS DataBase Reference | 74-93-1(CAS DataBase Reference) | | EPA Substance Registry System | Methyl mercaptan (74-93-1) |
| Hazard Codes | F+,T,N | | Risk Statements | 12-23-50/53 | | Safety Statements | 16-25-60-61 | | RIDADR | UN 2037 2.3 | | OEL | Ceiling: 0.5 ppm (1 mg/m3) [15-minute] | | WGK Germany | 3 | | RTECS | PB4375000 | | F | 13-27 | | HazardClass | 2.3 | | Hazardous Substances Data | 74-93-1(Hazardous Substances Data) | | Toxicity | LC50 (inhalation) for mice 6,530 μg/m3/2-h, rats 675 ppm (quoted, RTECS, 1985). | | IDLA | 150 ppm |
| | METHYL MERCAPTAN Usage And Synthesis |
| Description | Methanethiol (also known as methyl mercaptan) is a colorless gas with a smell like rotten cabbage. It is a natural substance found in the blood and brain of humans and other animals as well as plant tissues. It is disposed of through animal feces. It occurs naturally in certain foods, such as some nuts and cheese. It is also one of the main chemicals responsible for bad breath and the smell of flatus. The chemical formula for methanethiol is CH3SH; it is classified as a thiol. It is sometimes abbreviated as MeSH. It is very flammable. | | Chemical Properties | colourless gas with a garlic-like or rotten cabbage-like smell | | Chemical Properties | Methyl mercaptan has an objectionable odor of decomposing cabbage or garlic | | Chemical Properties | Methyl mercaptan is a colorless gas or white
liquid with a disagreeable odor like garlic or rotten cabbage. Shipped as a liquefied compressed gas. The odor
threshold is 0.002 ppm. | | Chemical Properties | Methyl mercaptan has an objectionable odor of decomposing cabbage. May be prepared by heating an aqueous solution of potassium methyl sulfate and KHS; from sodium methyl sulfate and
potassium sulfhydrate; also from methanol and hydrogen sulfide
in the presence of a catalyst. | | Physical properties | Colorless gas with a garlic-like or rotten cabbage odor. An experimentally determined odor
threshold concentration of 2.1 ppbv was reported by Leonardos et al. (1969). A detection odor
threshold concentration of 81 μg/m3 (41 ppbv) was determined by Katz and Talbert (1930). | | Occurrence | Methanethiol is released from decaying organic matter in marshes and is present in the natural gas of certain regions, in coal tar, and in some crude oils.
In surface seawater, methanethiol is the primary breakdown product of the algal metabolite dimethylsulfoniopropionate (DMSP). Marine bacteria appear to obtain most of their protein sulfur by the breakdown of DMSP and incorporation of methanethiol, despite the fact that methanethiol is present in seawater at much lower concentrations than sulfate (~0.3 nM vs. 28 mM). Bacteria in oxic and anoxic environments can also convert methanethiol to dimethyl sulfide (DMS), although most DMS in surface seawater is produced by a separate pathway. Both DMS and methanethiol can be used by certain microbes as substrates for methanogenesis in some anaerobic soils.
Methanethiol is a weak acid, with a pKa of ~10.4. This acidic property makes it reactive with dissolved metals in aqueous solutions. The environmental chemistry of these interactions in seawater or fresh water environments such as lakes has yet to be fully investigated.
A material safety data sheet (MSDS) lists methanethiol as a colorless, flammable gas with an extremely strong and repulsive smell. At very high concentrations it is highly toxic and affects the central nervous system. Its penetrating odor provides warning at dangerous concentrations. An odor threshold of 1 ppb has been reported. The United States OSHA Ceiling Limit is listed as 10 ppm. | | Uses | Methanethiol is mainly used to produce methionine, which is used as a dietary component in poultry and animal feed. Methanethiol is also used in the plastics industry and as a precursor in the manufacture of pesticides. It is released as a by-product of wood pulping in pulp mills.
Methanethiol is also used for communication in mining operations . Releasing the substance into the ventilation system is generally the most efficient and reliable means to alert all workers of an emergency , and is referred to as "releasing the pest" ,This substance's strong odor alerts the miners to immediately go to a saferoom.
Since natural gas and propane are colorless and odorless, a small amount of methyl mercaptan or ethyl mercaptan is added to make it easy to detect a gas leak. | | Uses | Methanethiol is used in the manufacture ofpesticides and fungicides and as an intermediate in the manufacture of jet fuels (Watkinset al. 1989); it is added to natural gas to giveodor. It is used in the synthesis of methionine. | | Uses | Methanethiol is an Intermediate in manufacturing of jet fuels, pesticides, fungicides, plastics; synthesis of methionine; emission from paper pulp mills, odoriferous additive to natural gas. | | Preparation | Methanethiol is prepared commercially by the reaction of methanol with hydrogen sulfide gas over an acidic solid catalyst, such as alumina. It can be prepared by the reaction of methyl iodide with thiourea. | | Definition | ChEBI: Methanethiol is an alkanethiol. It has a role as a human metabolite and a Saccharomyces cerevisiae metabolite. | | Aroma threshold values | Detection: 0.02 to 4 ppb. Aroma characteristics at 1.0%: vegetable oil, alliaceous, eggy, creamy with
savory nuances | | Taste threshold values | Taste characteristics at 1 ppm: sulfurous, alliaceous, creamy with a surface-ripened cheese top note and a
clean, savory, meaty depth | | Air & Water Reactions | Highly flammable. Reacts with water, steam or acids to produce toxic, flammable vapors [Lewis]. | | Reactivity Profile | METHYL MERCAPTAN is a reducing agent--can react vigorously with oxidizing agents. Dangerous fire or explosion hazard when exposed to heat, flame, sparks or strong oxidizing agents (e.g., calcium hypochlorite). When heating to decomposition emits highly toxic fumes of oxides of sulfur [Lewis, 3rd ed., 1993, p. 862]. Violent reaction with mercury(II) oxide [Klason P., Ber., 1887, 20, p. 3410]. | | Hazard | Flammable, dangerous fire risk. Explosive
limits in air 3.9–21.8%. Strong irritant. Liver damage. | | Health Hazard | Can cause death by respiratory paralysis. It is an eye and respiratory tract irritant. Exposure results in pulmonary edema and hepatic and renal damage. | | Health Hazard | The acute toxicity of methanethiol is simi-lar to that of hydrogen sulfide. Inhalation ofthis gas can cause narcosis, headache, nau-sea, pulmonary irritation, and convulsions inhumans. Other symptoms noted are acutehemolytic anemia, methemoglobinemia, andcyanosis. In humans, several hours exposure to about 5 ppm concentration of thisgas can cause headache and nausea. Exposure to high concentrations can result inrespiratory paralysis and death. The 2-hour inhalation LC50 value in mice is within therange 650 mg/m3.Shults et al. (1970) reported a case of ahuman death from overexposure to methane-thiol. The victim developed acute hemolyticanemia and methemoglobinemia and re-mainedindeepcomauntil deaththatoccurred28 days after the accident from emptying gascylinders. | | Fire Hazard | Combustion produces irritating sulfur dioxide. Flash back along vapor track may occur. Very dangerous when exposed to heat, flame, or oxidizers. On decomposition METHYL MERCAPTAN emits highly toxic fumes of sulfur oxides. METHYL MERCAPTAN will react with water, steam or acids to produce toxic and flammable vapors; and can react vigorously with oxidizing materials. Irritating sulfur dioxide is produced upon combustion. When heated to decomposition, METHYL MERCAPTAN emits highly toxic fumes and flammable vapors. Incompatible with mercuric oxide and oxidizing materials. Avoid direct sunlight, and areas of high fire hazards. Hazardous polymerization may not occur. | | Safety Profile | Poison by inhalation.
Mutation data reported. A common air
contaminant. Very dangerous fire hazard
when exposed to heat or flame; can react
vigorously with oxidzing materials.
Explosive in the form of vapor when
exposed to heat or flame. Reacts with water,
steam, or acids to produce toxic and
flammable vapors. Violent reaction with
mercury(II) oxide. To fight fire, use alcohol
foam, CO2, dry chemical. Upon
decomposition it emits highly toxic fumes of
SOx. | | Synthesis | By heating an aqueous solution of potassium methyl sulfate and KHS; from sodium methyl sulfate and potassium sulfhy�drate; also from methanol and hydrogen sulfide in the presence of a catalyst | | Potential Exposure | Methyl mercaptan is used in methionine synthesis, and widely as an intermediate in pesticide
manufacture. A foul-smelling odorant usually added to
chemicals, including pesticides. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at leastIf this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least | | Source | Occurs naturally in kohlrabi stems (Brassica oleracea var. gongylodes) and potato plants
(Duke, 1992) | | Environmental fate | Biological. After 20 d, methyl mercaptan started to degrade in anaerobic sediments and sludges
producing stoichiometric amounts of methane. Complete degradation was achieved after 20 d. Under
anaerobic freshwater conditions, methyl mercaptan were degraded by methanogenic archea (van
Leerdam et al., 2006).
Photolytic. Sunlight irradiation of a methyl mercaptan-nitrogen oxide mixture in an outdoor
chamber yielded formaldehyde, sulfur dioxide, nitric acid, methyl nitrate, methanesulfonic acid,
and an inorganic sulfate (Grosjean, 1984a).
Chemical/Physical. In the presence of nitric oxide, gaseous methyl mercaptan reacted with OH
radicals forming methyl sulfenic acid and methyl thionitrite. The rate constant for this reaction is
2.1 x 10-11 cm3/molecule?sec at 20 °C (MacLeod et al., 1984).
Forms a crystalline hydrate with water (Patnaik, 1992). | | storage | Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Methylmercaptan must be stored to avoid contact with water,steam, or strong acids (such as hydrochloric, sulfuric, andnitric) because toxic flammable vapors will be released. Itshould not contact oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates) since violentreactions occur. Store in tightly closed containers in a cool,well-ventilated area away from heat or sparks. Sources ofignition, such as smoking and open flames, are prohibitedwhere methyl mercaptan is handled, used, or stored. Metalcontainers involving the transfer of 5 gallons or more ofmethyl mercaptan should be grounded and bonded. Drumsmust be equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof methyl mercaptan. Procedures for the handling, use, andstorage of cylinders should be in compliance with OSHA1910.101 and 1910.169, as with the recommendations ofthe Compressed Gas Association. | | Shipping | UN1064 Methyl mercaptan, Hazard Class: 2.3;
Labels: 2.3-Poisonous gas, 2.1-Flammable gas, Inhalation
Hazard Zone C. Cylinders must be transported in a secure
upright position, in a well-ventilated truck. Protect cylinder
and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal
law (49CFR) to transport and refill them. It is a violation
of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. | | Asparagus | Methanethiol is a byproduct produced by the metabolism of asparagus. The ability to produce methanethiol in urine after eating asparagus was once thought to be a genetic trait. However recent research suggests that the peculiar odor is in fact produced by all humans after consuming asparagus, while the ability to detect it (methanethiol being one of many components in "asparagus pee") is in fact the genetic trait. The chemical components responsible for the change in the odor of urine show as soon as 15 minutes after eating asparagus. | | Incompatibilities | Violent reaction with strong oxidizers,
bleaches, copper, nickel and their alloys; aluminum. Reacts
with acids producing flammable and toxic hydrogen
sulfide | | Toxics Screening Level | The initial threshold screening level (ITSL) for Methyl mercaptan is 10 μg/m3 (1 hr.averaging) based on a NIOSH 15-min. | | Waste Disposal | Return refillable compressed
gas cylinders to supplier. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal.
Incineration followed by effective scrubbing of the effluent
gas. |
| | METHYL MERCAPTAN Preparation Products And Raw materials |
|