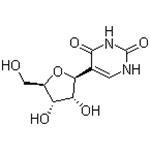
- Pseudouridine
-
- $0.00 / 10g
-
2025-04-29
- CAS:1445-07-4
- Min. Order: 10g
- Purity: 99% HPLC
- Supply Ability: 10000
- Pseudouridine
-

- $0.00 / 1g
-
2025-04-28
- CAS:1445-07-4
- Min. Order: 1g
- Purity: 98%min
- Supply Ability: 1000g
- Pseudouridine
-

- $999.00/ kg
-
2025-04-27
- CAS:1445-07-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
|
| | Pseudouridine Basic information |
| | Pseudouridine Chemical Properties |
| Melting point | 222 °C | | density | 1.641±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Methanol (Very Slightly, Heated), Water (Slightly, Sonicated, Heated) | | pka | 8.52±0.10(Predicted) | | form | Solid | | color | White to Off-White | | PH | ~9.0 | | biological source | synthetic (chemical) | | Water Solubility | water: soluble | | λmax | 264nm(MeOH)(lit.) | | InChI | InChI=1/C9H12N2O6/c12-2-4-5(13)6(14)7(17-4)3-1-10-9(16)11-8(3)15/h1,4-7,12-14H,2H2,(H2,10,11,15,16)/t4-,5-,6-,7+/s3 | | InChIKey | PTJWIQPHWPFNBW-IZFRNLNUNA-N | | SMILES | O[C@@H]1[C@@H]([C@@H](CO)O[C@H]1C1=CNC(=O)NC1=O)O |&1:1,2,3,7,r| |
| | Pseudouridine Usage And Synthesis |
| Description | β-Pseudouridine is the C-5 glycoside isomer of the nucleoside uridine. It is formed when uridine in RNA undergoes site-specific isomerization by a pseudouridine synthase enzyme. Pseudouridine is found in tRNAs from bacteria, archaea, and eukaryotes. In vitro, it reduces the number of X-ray-induced chromosomal aberrations in human lymphocytes isolated from whole blood in a dose-dependent manner. | | Uses | An isomer of the nucleoside uridine found in all species and in many classes of RNA except mRNA. It is formed by enzymes called Ψ synthases, which post-transcriptionally isomerize specific uridine residues in RNA in a process termed pseudouridylation. Studies suggest that β-Pseudouridine reduces radiation-induced chromosome aberrations in human lymphocytes. | | Definition | ChEBI: Pseudouridine is a C-glycosyl pyrimidine that consists of uracil having a beta-D-ribofuranosyl residue attached at position 5. The C-glycosyl isomer of the nucleoside uridine. It has a role as a fundamental metabolite. | | Origin | Pseudouridine (Ψ) was the first modified ribonucleotide discovered 7 decades ago, and it has been found in tRNA, rRNA, snRNA, mRNA, and other types of RNA[2]. | | Biochem/physiol Actions | Pseudouridine is essential for rRNA folding and for regulating translational accuracy and it is required for stabilizing the tRNA structure. Pseudouridine in rRNA and tRNA has been shown to fine-tune and stabilize the regional structure and help maintain their functions in mRNA decoding, ribosome assembly, processing and translation. Pseudouridine in snRNA has been shown to enhance spliceosomal RNA-pre-mRNA interaction to facilitate splicing regulation[1]. | | target | Human Endogenous Metabolite | | Structure and conformation | Pseudouridine, being one of them, is the C5-glycoside isomer of uridine that contains a C-C bond between C1 of the ribose sugar and C5 of uracil, rather than usual C1-N1 bond found in uridine. The C-C bond gives it more rotational freedom and conformational flexibility.In addition, pseudouridine has an extra hydrogen bond donor at the N1 position. | | References | [1] Mingjia Chen, Claus-Peter Witte. “A Kinase and a Glycosylase Catabolize Pseudouridine in the Peroxisome to Prevent Toxic Pseudouridine Monophosphate Accumulation.” Plant Cell 32 3 (2020): 722–739.
[2] Pedro Morais, Yi-Tao Yu, Hironori Adachi. “The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines.” Frontiers in Cell and Developmental Biology (2021): 789427. |
| | Pseudouridine Preparation Products And Raw materials |
| Raw materials | 2,4(1H,3H)-Pyrimidinedione, 5-[5-O-[(1,1-dimethylethyl)diphenylsilyl]-2,3-O-(1-methylethylidene)-β-D-ribofuranosyl]--->D-Ribitol, 1,4-anhydro-1-C-(2,4-dimethoxy-5-pyrimidinyl)-, (1S)--->α-Pseudouridine-->2,4(1H,3H)-Pyrimidinedione, 5-[2,3-O-(1-methylethylidene)-β-D-ribofuranosyl]--->2,4(1H,3H)-Pyrimidinedione, 5-[5-O-[(1,1-dimethylethyl)diphenylsilyl]-β-D-ribofuranosyl]--->5-O-(1-Methoxy-1-Methylethyl)-2,3-O-(1-Methylethylidene)-D-ribonic Acid γ-Lactone-->PSEUDOISOCYTIDINE-->D(+)-Ribonic acid gamma-lactone |
|