- Lovastatin
-

- $0.00 / 1KG
-
2025-04-24
- CAS:75330-75-5
- Min. Order: 1KG
- Purity: 0.02%-2% HPLC
- Supply Ability: 1000KG
- Monacolin K
-

- $0.00 / 1kg
-
2025-04-24
- CAS:75330-75-5
- Min. Order: 1kg
- Purity: 0.02%-2% HPLC
- Supply Ability: 1000kg
- Lovastatin
-

- $0.00 / 25Kg/Drum
-
2025-04-24
- CAS:75330-75-5
- Min. Order: 1KG
- Purity: 98.5%min USP
- Supply Ability: 500kg
|
| Product Name: | Lovastatin | | Synonyms: | SiMvastatin EP IMpurity E;8-[2-(4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-nap;6alpha-Methylcompactin
Mevinolin
Monacolin K;)ethyl]-1-naphthalenylester;[1s-[1alpha(r*),3alpha,7beta,8beta(2s*,4s*),8abeta]]-2-methylbutanoicacid1,2,;1,2,6,7,8,8a-hexahydro-beta,delta-dihydroxy-2,6-dimethyl-8-(2-methyl-1-oxobuty;2-methyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-butanoicaci;3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2h-pyran-2-yl | | CAS: | 75330-75-5 | | MF: | C24H36O5 | | MW: | 404.54 | | EINECS: | 616-212-7 | | Product Categories: | Inhibitor;Heterocycles;chemical reagent;Inhibitors;Cardiovascular APIs;antibiotic;RYTHYMOL;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Isotopically Labeled Pharmaceutical Reference Standard;API's;Intermediates & Fine Chemicals;Pharmaceuticals;APIs;Antibiotics;HMG-CoA reductase;Chiral Reagents;75330-75-5 | | Mol File: | 75330-75-5.mol | 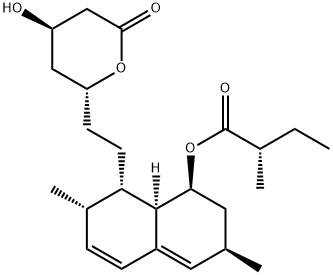 |
| | Lovastatin Chemical Properties |
| Melting point | 175°C | | alpha | D25 +323° (c = 0.5 g in 100 ml acetonitrile) | | Boiling point | 559.2±50.0 °C(Predicted) | | density | 1.12±0.1 g/cm3(Predicted) | | vapor pressure | 0Pa at 25℃ | | refractive index | 320 ° (C=0.5, CH3CN) | | storage temp. | -20°C | | solubility | ethanol: soluble9.80-10.20mg/mL, clear, colorless to faintly yellow | | pka | 13.49±0.40(Predicted) | | form | White to off-white powder | | color | White | | Water Solubility | 0.0004 mg/mL at 25 ºC | | Merck | 14,5586 | | BCS Class | 2 | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. | | LogP | 4.260 | | CAS DataBase Reference | 75330-75-5(CAS DataBase Reference) | | EPA Substance Registry System | Lovastatin (75330-75-5) |
| | Lovastatin Usage And Synthesis |
| a cholesterol lowering agent | Lovastatin is a cholesterol lowering agent isolated from a strain of Aspergillus terreus.
Lovastatin is a white, nonhygroscopic crystalline powder that is insoluble in water and sparingly soluble in ethanol, methanol, and acetonitrile.
After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the corresponding β-hydroxyacid form. This is a principal metabolite and an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, which is an early and rate limiting step in the biosynthesis of cholesterol.
| | General description | Lovastatin is a hexahydro naphthalene ester isolated from Aspergillus terreus broth. It is an inactive lactone only yielding activity upon hydrolysis after oral administration. It has a strong competitive inhibition on the HMG-CoA reductase in the liver. HMG-CoA reductase is rate-limiting enzyme for de novo cholesterol synthesis in vivo. The inhibition of the enzyme can block the conversion of HMG-CoA to methacrylic acid, causing significant reduction in cholesterol synthesis, and leading to increased expression of the liver LDL receptor which promotes the plasma LDL-C clearance. The reduction of cholesterol synthesis can also cause reduced synthesis of liver ApoB100, thus causing that reduction of VLDL synthesis. Clinical observations show that it has a good effect of lowering of plasma total cholesterol and LDLC on hypercholesterolemia caused by various kinds of reasons such as heterozygous familial hypercholesterolemia, polygenic hypercholesterolemia, and diabetes or kidney disease syndrome.
Statins are the secondary metabolites of filamentous fungi. It is capable of selectively inhibiting the activity of HMG coenzyme A (HMG-CoA) reductase and blocking cholesterol biosynthesis, which is due to the similarity of its acid structure with HMG-CoA. These drugs currently used in humans (statin drugs, statins) are mainly either natural or synthetic statins. Natural statins include lovastatin, mevastatin, Pravastatin and simvastatin. Lovastatin is produced by the fermentation of Aspergillus terreus); mevastatin is produced through the fermentation of penicillium citrinum and further conversion by streptomyces carbophilus; Simvastatin is made by semi-synthesis (chemically modified side chains) through lovastatin. Synthetic statins include fluvastatin, cerivastatin and atorvastatin.
| | Structure of statin compound | Natural statin compounds is has very similar structure with each other. They have the same poly-ketone portion of hydroxy-hexahydro-cyclic with different side chains attached to C8 (R1) and C6 (R2) bits. R1 of lovastatin is methylbutyryl, R2 is 6-α-methyl; mevastatin has no 6-position methyl. Natural statin compounds are all in the form of lactone. They need to be hydrolyzed to acidic form before becoming active. Fully synthetic statin compound, although is structurally different from the natural statins but still have a lactone ring-opening portion which is the common structure of all statins which is responsible for its competitive inhibition on HMG-CoA reductase.
| | Active Mechanism | There are two main sources of plasma cholesterol; one is exogenous cholesterol absorbed from dietary; the other is form endogenous biosynthesis in vivo, wherein the endogenous cholesterol accounts for 2/3 of the total cholesterol which makes it the primary target of lipid-lowering therapy.
Cholesterol biosynthetic pathway in vivo starts from acetyl coenzyme. HMG-CoA is first generated by the HMG-CoA synthase, and then reduced into mevalonate by HMG-CoA reductase; then went through phosphorylation to generates farnesyl pyrophosphate; further reduced to turtle-ene which finally converts to cholesterol through 20 steps including lanosterol and chain sterols, wherein the conversion of HMG-CoA to mevalonate via HMG-CoA reductase is the rate-limiting step in cholesterol synthesis, making HMG-CoA reductase is the rate-limiting enzyme. Therefore, the inhibition of HMG-CoA reductase activity can reduce the formation of endogenous cholesterol. A part of lovastatin structure, 3,5-dihydroxy heptanoic acid is quite similar with HMG-CoA and its inhibitory effect is 10,000 times higher than HMG-CoA intermediate so that it can competitively bind with HMG-CoA reductase to inhibit the synthesis of mevalonate lactone, and thus effectively reducing the speed of cholesterol synthesis in liver cells and ultimately inhibiting the biosynthesis of endogenous cholesterol.
| | Medicinal Value | 1. Regulation of Lipid Metabolism
Lovastatin inhibit the endogenous cholesterol synthesis by inhibiting its rate-limiting enzyme, HMG-CoA reductase. It lowers the intracellular cholesterol level and increases the number of LDL receptors on the cell surface through feedback action, thus accelerating the uptake and degradation of LDL particles in blood circulation and reducing the contents of total cholesterol and very low density lipoprotein (VLDL), LDL and triglycerides. Since the conversion of HMG-CoA into mevalonate is an early step in the cholesterol biosynthetic pathway, the use of lovastatin doesn’t cause accumulation of potentially toxic cholesterol-class substances. Moreover, HMG-CoA can quickly reverse metabolized to acetyl coenzyme A and participate in other biosynthetic pathways in vivo. The inhibition is incomplete, reversible and has dose-effect dependence. The therapeutic doses does not affect the amount of cholesterol required for normal function of cell membranes, therefore, lovastatin has a significant lipid-lowering effects with small side effects.
Overall, lovastatin takes effect mainly by the following aspects: (1) competitive inhibition of HMG-CoA reductase activity, reduction of endogenous cholesterol synthesis; (2) Increase the expression of LDL receptor in liver cells, enhance receptor-mediated plasma LDL clearance rate; (3) inhibit the migration and proliferation of smooth muscle cell; (4) reduce the assembly and secretion of lipoproteins in the liver.
2. Non-lipid function
In addition to its significant lipid-lowering effect, lovastatin can also improve endothelial function, promote the synthesis of nitric oxide synthase (eNOS), thereby increasing the synthesis and release of NO, which is crucial for the maintenance of normal human pulmonary artery tension and reversing the hypoxia-induced pulmonary vasoconstriction and vascular remodeling.
In addition, lovastatin has anti-inflammatory and anti-proliferative effect. It can inhibit the mesangial cell proliferation and secretion of extracellular matrix for achieving the purpose of alleviating glomerulosclerosis.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Biosynthesis | Acetate and propionic acid are went through condensation, reduction, dehydration to form diketone intermediate, the process is catalyzed by keto reductase (KR), alcohol reductase (ER) or methyltransferase (Met) and repeated once to form hexanone followed by the enzymatic Diels-Alder reaction to produce the skeleton of dicyclo-decalin. The dicyclo adduct is further extended into nine-ketone which is releases from polyketone synthase (PKS) to form 4a, 5-dihydro Monacolin L, 4a, 5-dihydro Monacolin L when can be converted to hydroxy-3,5-dihydro-3α-Monacolin L in the presence of molecular oxygen. The latter one can be spontaneously dehydrogenated to be converted into Monacolin L. Monacolin L, in the presence of molecular oxygen, has its C-8 be hydroxylated to become Mo Lin J. Inhibitory tests using metyrapone, carbon monoxide, and thiol agents proved that the enzyme involved in this reaction is a single oxygen dioxygenase. Monacolin J is esterified into lovastatin via (2R)-methylbutyrate.
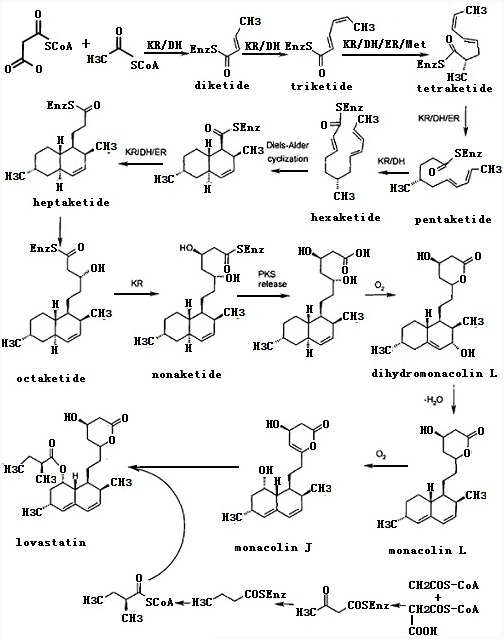
Figure 1 the synthesis route of lovastatin | | Uses | It is used for treating heterozygous familial and non familial, secondary hyperlipidemia, namely diabetes and nephrotic syndrome secondary hypercholesterolemia. It can reduce the level of TC, LF, LDL-C, and increase the level of HDL-C, reduce the risk of myocardial infarction, unstable angina and the necessity for percutaneous transluminal coronary angioplasty (PTCA).
| | Metabolism | This product goes through gastrointestinal absorption after oral administration, F is 30%, and increases to 50% when taken together with food F; Tmax: 2~4h; T1/2: 3h, the original drug and metabolites PPB> 95%; it can penetrate the blood brain barrier and the placental barrier. It mainly metabolized by liver with the metabolic enzyme being CYP3A4. 60%~83% of it is excreted via bile, and 10% to 13% via the urinary excretion.
| | Precautions | Patients of being allergic to this drug, active hepatitis or unexplained elevated serum transaminases are forbidden to use, and so are the pregnant and lactating women. Patients of renal insufficiency or renal transplant should take with caution.
Drugs which can inhibit the CYP3A4 enzyme such as macrolide antibiotics, benzodiazepines class, phenoxy acids, and niacin cholesterol lowering agents, cimetidine and a large grapefruit juice can all increase the plasma concentration of lovastatin and its metabolites and increase the risk of rhabdomyolysis.
Check the liver function after treatment or increasing the dose for 6 to 12 weeks, and check once every six months in future. Check CPK in cases such as muscle pain symptoms, and stop using if CPK level reaches a level 10 times of the normal level.
| | Adverse reaction | Adverse reactions are mild, rate, transient, such as headache, fatigue, gastrointestinal reactions (bloating, constipation, diarrhea, abdominal pain, nausea, indigestion, etc.), and skin rash. In occasion case, there are occurrence of decrease in white blood cells, thrombocytopenia, and abnormal liver function.
| | Chemical property | White crystals, melting point: 174.5 °C (nitrogen). [α] D25 + 323 °(C = 0.5g, dissolved in 100ml acetonitrile). UV absorption maximum (methanol): 229,237,246nm (three ears 550, 6250, 430). Solubility at RT (mg /mL): acetone: 47, acetonitrile: 28, n-butanol: 7, i-butanol: 14, chloroform: 350, dimethylformamide: 90, ethanol: 16, methanol: 28, n-octanol: 2, n-propanol: 11, i-propanol: 20, water 0.4 × 10-3. Acute toxicity LD50 in mice (mg/kg): > 1000 Oral.
| | Uses | 1. It is as a novel lipid regulating drugs-The inhibitor of HMG-CoA (β-hydroxy, β-methyl-glutaryl coenzyme A) reductase. It can significantly reduced serum total cholesterol level. After oral administration, it is hydrolyzed into corresponding β-hydroxy acid by 3-hydroxy-3-methylglutaryl coenzyme A reductase, and thus inhibiting the cholesterol biosynthesis. It is clinically used for treating heterozygous familial hypercholesterolemia, severe or mild hypercholesterolemia and light. It can also as an ancillary drug of dietary treatment for reducing the levels of excessively cholesterol and low-density protein cholesterol.
2. It is a cardiovascular systematic drug which can prevent the development of atherosclerosis and reduce the risk of myocardial infarction.
| | Production method | Lovastatin is produced by fermentation. Available species include: 1. Monescus ruber; 2. Monescus purpureus; 3. Monescus pilosus; 4. Aspergillus terreus; 5. Penicillium Citrunum.
When using Monescus ruber as the strain, the culture condition is as follows: 6% glucose, 2.5% peptone, 0.5% corn syrup, and 0.5% ammonium chloride. Strain in broth is grown in aerobic conditions at 28 °C for 10d. Filter and take 5 L filtrate; Use ethyl acetate (pH 3.0) for extraction. The extract was vacuum concentrated to dryness with the residue being dissolved in 100ml of benzene. Insoluble substances are further removed by filtration, wash the filtrate with 100ml 5% aqueous sodium carbonate twice, and then stir together with 100 mL 0.2mol/L sodium hydroxide solution at room temperature for 2h. The aqueous layer is collected, and be treated with 6 mol/L hydrochloric acid for adjusting pH to 3.0; Extract for twice with 100ml of ethyl acetate. Combine the extract and evaporate to dryness to give 260 mg oil. Dissolve oil-like product in a small amount of benzene; the obtained crystal is further re-crystallized by the mixture of acetone and water to give 87 mg of colorless lovastatin crystals, m.p. 157~159 °C (decomposition), [α] D23 + 307.6 ° (C = 1, methanol).
| | Description | Lovastatin is an orally-active hypocholesterolemic useful in the treatment of familial
hypercholesterolemia. The drug acts by inhibiting the HMG-CoA reductase-catalyzed rate
limiting step of cholesterol biosynthesis. While treatment with lovastatin leads to
significant reductions in total and LDL cholesterol, its effect on atherosclerosis or
coronary heart disease has not been established. | | Chemical Properties | White Solid | | Physical properties | Appearance: White, nonhygroscopic crystalline powder. Solubility: Insoluble in
water (0.0004?mg/mL); sparingly soluble in ethanol, methanol, isobutanol, isopropanol, acetonitrile, n-propanol; soluble in acetone, N-dimethylformamide; freely
soluble in chloroform. Melting point: 174.5?°C. Specific optical rotation: 25?°C for
D (sodium) line, +323° (concentration 0.5? g in 100? ml acetonitrile). Stability:
Lovastatin is sensitive to light. Following exposure to extreme light conditions, the
drug is stable for 24?h or 1?month when exposed to UV (approximately 3230 lux) or
fluorescent (approximately 10,764 lux) light, respectively, at 28?°C in air. | | Originator | Merck (USA) | | History | Statins are the most extensively used class of lipid-lowering medication. Lovastatin
is the second statin discovered by scientists.
Later, the official name lovastatin was established. The activity of lovastatin is
much better than compactin.
In July 1982, lovastatin showed dramatic effects on lowering LDL cholesterol in
patients with severe hypercholesterolemia who were unresponsive to the existedmedicines, with very few adverse reactions . | | Uses | Cardiovascular | | Uses | anti-hyperlipoproteinemic, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor | | Uses | antiarrhythmic | | Uses | Lovastatin (mevinolin) is a metabolite first isolated from Monascus ruber and later found in several other fungal species. Lovastatin is a potent inhibitor of HMG-CoA. HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, responsible for the biosynthesis of cholesterol. Lovastatin was developed as a drug as a hypolipemic agent. | | Uses | An antihypercholesterolemic agent. A fungal metabolite, which is a potent inhibitor of HMG-CoA reductase | | Indications | Hypercholesterolemia, combined hyperlipidemia. | | Definition | ChEBI: Lovastatin is a fatty acid ester that is mevastatin carrying an additional methyl group on the carbobicyclic skeleton. It is used in as an anticholesteremic drug and has been found in fungal species such as Aspergillus terreus and Pleurotus ostreatus (oyster mushroom). It has a role as an Aspergillus metabolite, a prodrug, an anticholesteremic drug and an antineoplastic agent. It is a polyketide, a statin (naturally occurring), a member of hexahydronaphthalenes, a delta-lactone and a fatty acid ester. It is functionally related to a (S)-2-methylbutyric acid and a mevastatin. | | Manufacturing Process | 1) Coniothyrium fuckelii ATCC 74227 was grown in a sterilizable fermentation apparatus with a volume of 15 L. The apparatus was equipped with an agitator, aerator, pH control system, dissolved oxygen control system, and a pump and feed system designed to allow the sterile addition of glucose solutions. The pH was controlled by the automatic addition of ammonium hydroxide or phosphoric acid to maintain the pH of the culture medium constant at 5.0. Periodically, the fermentation broth was sampled, measured for glucose concentration and an addition of glucose was made manually to maintain a concentration of glucose at approximately 2-5 g/L. After 192 hours of growth under these conditions, the concentration of biomass reached 65 g/L and the concentration of Lovastatin reached 102 mg/L.
2) A further medium for the growth of Coniothyrium fuckelii ATCC 74227, has the following composition: Glucose 12%, Peptone 1%, (NH4)2SO4 0.4%, MgSO4 · 7H2O 0.05%, P 2000 0.1% (Antifoam agent), L-isoleucine 0.2-1.5%, L-aspartic acid 0.2-1.5%. The fermentation was carried out as before.
With this medium, the lovastatin concentration was 430 mg/L. | | Brand name | Altoprev (Andrx); Mevacor
(Merck). | | Therapeutic Function | Antihyperlipidemic | | General Description | Lovastatin, 2-methylbutanoic acid 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester, mevinolin,MK-803 (Mevacor) (formerly called mevinolin), is a potentinhibitor of HMG-CoA. The drug was obtained originallyfrom the fermentation products of the fungi Aspergillus terreusand Monascus ruber. Lovastatin was one of two originalHMG-CoA reductase inhibitors. The other drug, mevastatin(formerly called compactin), was isolated from cultures ofPenicillium cillium citrum. Mevastatin was withdrawn fromclinical trials because it altered intestinal morphology in dogs.This effect was not observed with lovastatin. For inhibitoryeffects on HMG-CoA reductase, the lactone ring must be hydrolyzedto the open-ring heptanoic acid. | | Biological Activity | Potent, competitive inhibitor of HMG-CoA reductase (K i = 0.6 nM) therefore decreases cholesterol biosynthesis, in vitro and in vivo . Decreases CDK2, 4, 6 and cyclin E levels and induces G1 arrest and apoptosis in tumor cell lines in vitro . | | Pharmacology | Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
(HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to
mevalonate , a rate-determining step of cholesterol biosynthesis. Lovastatin is
metabolized as a prodrug into an active form, lovastatin acid. Lovastatin acid is a
reversible competitive inhibitor for HMG-CoA.
However, the reduction in plasma cholesterol by statins is not only due to reduction in cholesterol biosynthesis. .
In addition to their lipid-lowering properties, statins produce several nonlipidrelated properties, include decreasing levels of high-sensitivity C-reactive protein
(hsCRP), improving endothelial function, reducing inflammation at the site of the
atherosclerotic plaque, inhibiting platelet aggregation, anticoagulant, etc. . | | Clinical Use | The primary uses of lovastatin are the treatment of dyslipidemia and the prevention
of cardiovascular disease. It is recommended to be used only when other approaches
such as diet, exercise, and weight reduction have not improved the cholesterol profile (“Lovastatin”. The American Society of Health-System Pharmacists. Retrieved
3 April 2011.). Lovastatin is useful in treating hypercholesterolemia and combined
hyperlipidemia. However, lovastatin is not effective in treatment of receptornegative homozygous familial hypercholesterolemia . | | Side effects | Lovastatin's common side effects are listed in approximately descending order of occurrence frequency: creatine phosphokinase elevation, flatulence, abdominal pain, constipation, diarrhea, muscle aches or pains,
nausea, indigestion, weakness, blurred vision, rash, dizziness, and muscle cramps.
As with all statin drugs, it can rarely cause myopathy, hepatotoxicity (liver damage),
dermatomyositis, or rhabdomyolysis. | | storage | Store at RT | | References | 1) Alberts et al. (1988), Discovery, biochemistry and biology of lovastatin;? Am. J. Cardiol., 62 10J
2) Hancock et al. (1989), All ras proteins are polyisoprenylated but only some are palmitoylated;? Cell, 57 1167
3) Park et al. (1999), Lovastatin-induced inhibition of HL-60 cell proliferation via cell cycle arrest and apoptosis;? Anticancer Res., 19 3133
4) Vilimanovich et al. (2015), Statin-mediated inhibition of cholesterol synthesis induces cytoprotective autophagy in human leukemic cells;? Eur. J. Pharmacol., 765 415
5) Tobert et al. (1988), Efficacy and long-term adverse effect pattern of lovastatin;? Am. J. Cardiol., 62 28J |
| | Lovastatin Preparation Products And Raw materials |
|