| Company Name: |
Lynnchem
|
| Tel: |
86-(0)29-85992781 17792393971 |
| Email: |
info@lynnchem.com |
| Products Intro: |
Product Name:PlatensiMycin
CAS:835876-32-9
Purity:>95% by HPLC Package:1mg;5mg
|
| Company Name: |
Novachemistry
|
| Tel: |
44-20819178-90 02081917890 |
| Email: |
info@novachemistry.com |
| Products Intro: |
Product Name:Platensimycin
CAS:835876-32-9
Purity:>95% by HPLC Package:1mg;5mg
|
|
| | PlatensiMycin Basic information |
| Product Name: | PlatensiMycin | | Synonyms: | PlatensiMycin;Platensimycin, Streptomyces sp. - CAS 835876-32-9 - Calbiochem;Benzoic acid, 3-[[3-[(1S,3S,4S,5aS,9S,9aR)-1,4,5,8,9,9a-hexahydro-3,9-dimethyl-8-oxo-3H-1,4:3,5a-dimethano-2-benzoxepin-9-yl]-1-oxopropyl]amino]-2,4-dihydroxy-;laminomycin | | CAS: | 835876-32-9 | | MF: | C24H27NO7 | | MW: | 441.474 | | EINECS: | | | Product Categories: | | | Mol File: | 835876-32-9.mol | 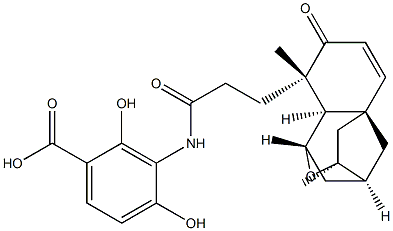 |
| | PlatensiMycin Chemical Properties |
| Melting point | 220-222 °C(Solv: nitromethane (75-52-5)) | | Boiling point | 683.1±55.0 °C(Predicted) | | density | 1.47±0.1 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | DMSO: soluble1mg/mL, clear, colorless | | pka | 2.14±0.13(Predicted) | | form | Tan solid | | color | white to beige |
| | PlatensiMycin Usage And Synthesis |
| Uses | Platensimycin is a broad spectrum gram-positive antibiotic. | | Uses | Platensimycin is a novel, broad spectrum, Gram-positive antibiotic produced by strains of Streptomyces platensis. Its discovery was heralded by high profile publication and commentary in the scientific and lay press. Platensimycin was discovered by a target-based, whole-cell screening strategy using an antisense differential sensitivity assay, based on the inhibition of fatty acid synthesis. Platensimycin inhibits bacterial growth by selectively inhibiting the elongation enzyme, b-ketoacyl acyl carrier protein synthase (FabF) of the fatty acid synthesis pathway. | | Definition | ChEBI: A monocarboxylic acid amide obtained by the formal condensation of the amino group of 3-amino-2,4-dihydroxybenzoic acid with the carboxy group of the oxatetracyclic cage component. It is an antibiotic isolated from Streptomyces platensis a
d exhibits inhibitory activity against fatty acid synthase. | | Enzyme inhibitor | This thiol-reactive Streptomyces platensis-derived antibiotic (FW = 438.50 g/mol; CAS 835876-32-9), also named 3-[[3-[(1R,3R,4R,5aR,9R,9aS)- 1,4,5,8,9,9a-hexahydro-3,9-dimethyl-8-oxo-3H-1,4:3,5a-dimethano-2- benzoxepin-9-yl]-1-oxopropyl]amino]-2,4-dihydroxybenzoic acid, inhibits Staphylococcus aureus b-ketoacyl-[acyl-carrier-protein] synthase II, or FabF (IC50 = 290 nM). This enzyme, which allows bacteria to produce the fatty acids needed for making cell membranes, is thus a uniquely druggable prokaryotic target. Platensimycin has potent, broad-spectrum Gram-positive activity in vitro and exhibits no cross-resistance to other key antibiotic-resistant bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate S. aureus, vancomycin-resistant Enterococci, as well as linezolid-resistant and macrolide-resistant pathogens. Platencin is a more potent analogue of Platensimycin. While effective in vivo when continuously administered, its efficacy is reduced when administered by more conventional means. Mechanism of Action: The Cys163 within the FabF active site is activated through the dipole moment of helix N-a-3, lowering its pKa, also increasing its nucleophilicity by the stabilizing effects of FabF’s catalytically required oxyanion hole. Interestingly, the crystal structure complex with platensimycin employed a C163Q mutant which gave a 50-fold increase in apparent binding. |
| | PlatensiMycin Preparation Products And Raw materials |
|