|
|
| Product Name: | AlPhos | | Synonyms: | [4''-Butyl-2'',3'',5'',6''-tetrafluoro-3-methoxy-2',4',6'-tris(1-methylethyl)[1,1':3',1''-terphenyl]-2-yl]bis(tricyclo[3.3.1.1(3,7)]dec-1-yl)phosphine;6-Methoxy-2-{2,4,6-tri-i-propyl-3-[(2,3,5,6-tetrafluoro-4-butyl )phenyl]phenyl}diadamantyl phosphine, 98% AlPhos;6-Methoxy-2-{2,4,6-tri-i-propyl-3-[(2,3,5,6-tetrafluoro-4-butyl
)phenyl]phenyl}diadamantyl phosphine, 98%;2-(DIADAMANTYLPHOSPHINO)-3-METHOXY-2,4,6-TRI-I-PROPYL-3-(2,3,5,6-TETRAFLUORO-4-BUTYLPHENYL)-1,1-BIPHENYLALPHOS;6-Methoxy-2-{2,4,6-tri-i-propyl-3-[(2,3,5,6-tetrafluoro-4-butyl )phenyl]phenyl}diadamantyl phosphine, AlPhos;[4''-Butyl-2'',3'',5'',6''-tetrafluoro-3-methoxy-2',4',6'-tris(1-methylethyl)[1,1';3',1''-terphenyl]-2-yl]bis(tricyclo[3.3.1.1(3,7)]dec-1-yl)phosphine;bis(1-adamantyl)-[2-[3-(4-butyl-2,3,5,6-tetrafluorophenyl)-2,4,6-tri(propan-2-yl)phenyl]-6-methoxyphenyl]phosphane | | CAS: | 1805783-60-1 | | MF: | C52H67F4OP | | MW: | 815.06 | | EINECS: | | | Product Categories: | Buchwald Ligands Series;Buchwald Ligands&Precatalysts;Achiral Phosphine;Aryl Phosphine | | Mol File: | 1805783-60-1.mol | 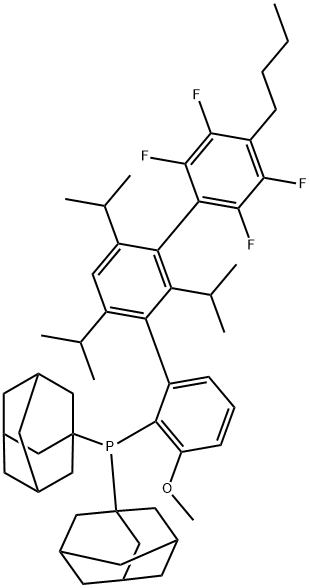 |
| | AlPhos Chemical Properties |
| Melting point | 218-223 °C | | storage temp. | -20°C | | form | Powder | | color | white to yellow | | Sensitive | air sensitive | | InChIKey | ALWIRDZSIXWCBO-UHFFFAOYSA-N | | SMILES | P(C1=C(OC)C=CC=C1C1=C(C(C)C)C=C(C(C)C)C(C2=C(F)C(F)=C(CCCC)C(F)=C2F)=C1C(C)C)(C12CC3CC(CC(C3)C1)C2)C12CC3CC(CC(C3)C1)C2 |
| | AlPhos Usage And Synthesis |
| Reaction |
- Ligand for the Palladium-Catalyzed Fluorination of Five-Membered Heteroaryl Bromides
- Ligand for the Palladium-Catalyzed Fluorination of Aryl Triflates and Bromides
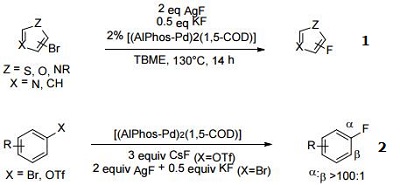
| | Uses | AlPhos is used in the selective arylation of aminophenylalanine in unprotected peptides with organometallic palladium reagents. | | General Description | AlPhos is a versatile, highly efficient ligand used extensively in various important organic transformations. Recognized for its unique structure and attributes, AlPhos serves as an impressive catalyst that enables several key synthetic processes. It is primarily used in the following coupling reactions:
Buchwald-Hartwig cross coupling, Suzuki-Miyaura coupling, Stille coupling, Sonogashira coupling, Negishi coupling, Heck coupling, Hiyama coupling reaction. With its remarkable performance across these reactions, AlPhos represents an invaluable tool in preparing and producing pharmaceuticals, agrochemicals, and polymers. | | reaction suitability | reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings |
| | AlPhos Preparation Products And Raw materials |
|