|
|
| Product Name: | 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione | | Synonyms: | 16A-HYDROXY-PREDNISONLONE;11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione;16 Alpha Hydroxprednisolone;11,16a,17a,21-Tetrahydroxypregna-1,4-diene-3,20-dione;Einecs 237-731-1;16A-HYDROXY-PREDNISOLONE;11a,16b,17,21-tetrahydroxy-pregna-1,4-diene-3,20-dione;11B,16A,17A,21-TETRAHYDROXYPREGNA-1,4-DIENE-3,20-DIONE | | CAS: | 13951-70-7 | | MF: | C21H28O6 | | MW: | 376.44 | | EINECS: | 237-731-1 | | Product Categories: | Pharmaceutical Raw Materials;Metabolites & Impurities;Intermediates & Fine Chemicals;Metabolites;Pharmaceuticals;Steroids;13951-70-7 | | Mol File: | 13951-70-7.mol | 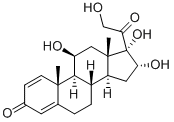 |
| | 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione Chemical Properties |
| Melting point | 235-238°C | | Boiling point | 591.5±50.0 °C(Predicted) | | density | 1.38±0.1 g/cm3(Predicted) | | vapor pressure | 0Pa at 25℃ | | storage temp. | Sealed in dry,2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly, Heated) | | form | Solid | | pka | 11.93±0.70(Predicted) | | color | White to Light Yellow | | Water Solubility | 815mg/L at 20℃ | | LogP | 0.8 at 25℃ | | CAS DataBase Reference | 13951-70-7(CAS DataBase Reference) |
| | 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione Usage And Synthesis |
| Related substances | Prednisolone is a synthetic adrenocortical steroid drug with predominantly glucocorticoid properties. | | Chemical Properties | White Crystalline Solid | | Uses | 11β,16α,17α,21-Tetrahydroxypregna-1,4-diene-3,20-dione (Budesonide EP Impurity A) is a metabolite of Budesonide (B689490), an anti-inflammatory agent. | | Uses | A metabolite of Budesonide (B689490), an antiinflammatory agent. | | Synthesis | 501 g of crude 16α-hydroxy prednisolone acetate is dissolved in 700 ml of dichloromethane and 700 ml of ethanol, then 1.5 ml of formic acid is added, 24 ml of 5% sodium hypochlorite aqueous solution is added dropwise, and the temperature is controlled at 15 °C, and the reaction is stirred to obtain the preliminary processed product; Add 150 ml of 5% sodium bisulfite aqueous solution to the above-mentioned preliminary treatment product, concentrate on removing the solvent, adding water to hydrolyze, and filter to obtain 45 g of 16α-hydroxyprednisolone acetate refined product; 45 g of 16α-hydroxyprednisolone acetate obtained above was dissolved in a mixed organic solvent of 250 ml of dichloromethane and 250 ml of methanol, stirred and cooled to 0 °C, 90 ml of 10% sodium sulfite aqueous solution was added, and the reaction was stirred. After completion, it was neutralized with 15% sulfuric acid by mass concentration; the mixed organic solvent was concentrated, watered out, filtered, and dried to obtain 36.5 g of 16α-hydroxyprednisolone (11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione).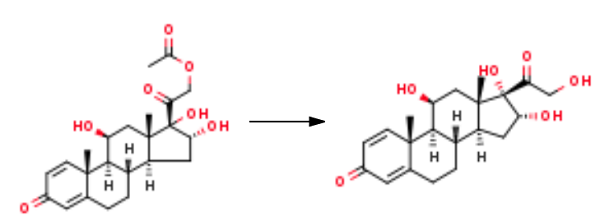 |
| | 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione Preparation Products And Raw materials |
|