| Company Name: |
ShangHai Angti Biotechnology Co., Ltd.
|
| Tel: |
13764913901 |
| Email: |
info@angtibio.com |
| Products Intro: |
Product Name:(3,3-Dimethoxyprop-1-en-1-yl)benzene 97% mix TBC as stabilizer
CAS:4364-06-1
|
|
| | ((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE Basic information |
| Product Name: | ((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE | | Synonyms: | (3,3-dimethoxy-1-propenyl)-benzen;(3,3-dimethoxy-1-propenyl)-Benzene;1,1-dimethoxy-3-phenylprop-2-ene;3-phenyl-2-propenaldimethylacetal;((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE;CINNAMIC ALDEHYDE DIMETHYL ACETAL;CINNAMALDEHYDE DIMETHYL ACETAL;beta-(dimethoxymethyl)styrene | | CAS: | 4364-06-1 | | MF: | C11H14O2 | | MW: | 178.23 | | EINECS: | 224-454-6 | | Product Categories: | | | Mol File: | 4364-06-1.mol | 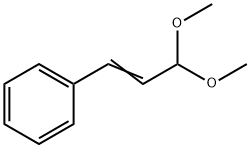 |
| | ((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE Chemical Properties |
| Boiling point | 270.46°C (rough estimate) | | density | 1.0292 (rough estimate) | | refractive index | 1.5103 (estimate) | | form | liquid | | Odor | at 10.00 % in dipropylene glycol. fresh green spicy cinnamon herbal | | Odor Type | spicy | | LogP | 2.689 (est) | | EPA Substance Registry System | Benzene, (3,3-dimethoxy-1-propenyl)- (4364-06-1) |
| toxicity | The acute oral LD50 value in rats was reported as 3.7 g/kg (3.3-4.1 g/kg) (Moreno, 1972a). The acute dermal LD50 value in rabbits exceeded 5 g/kg (Moreno, 1972b). |
| | ((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE Usage And Synthesis |
| Chemical Properties | Almost colorless oily liquid. B.P. 239° C.
Sp.Gr. 1.02. Fresh-green, spicy-herbaceous Cassia-cinnamon type odor, more Cassia-like than the
Diethyl acetal, yet less harsh than the aldehyde. Sweet spicy -herbaceous taste, but not nearly
as sweet as the alciehyde. | | Occurrence | Has apparently not been reported to occur in nature. | | Uses | (E)-Cinnamaldehyde Dimethyl Acetal is synthesized from trans-cinnamaldehyde (C442020) and is used for the preparation of benzyl(aryl)triazoles and their selective human aromatase (cytochrome P 450 19A1) inhibitory activity. | | Uses | ((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE is used as a lasting topnote ingredient in
many types of “modem” fragrances, not only
in copies of successful French perfumes, but
also in general as a novelty along with aldehydic-floral or Oriental-spicy or sweet-woody
fragrance types. It forms interesting topnotes
with alifatic aldehydes in Rose-Jasmin fragrances, and gives pleasant variations of woody-Oakmoss types. Stable in soap and may introduce Cinnamon type odor when used at a sufficiently high
level of concentration. | | Preparation | By the interaction of cinnamic aldehyde and methanol in the presence of a catalyst or by treating cinnamic aldehyde with trimethyl orthoformate. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 35, p. 1962, 1970 DOI: 10.1021/jo00831a052 |
| | ((E)-3,3-DIMETHOXY-PROPENYL)-BENZENE Preparation Products And Raw materials |
|