- 1,2-Dibromopropane
-

- $10.00 / 1kg
-
2025-03-28
- CAS:78-75-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- 1,2-Dibromopropane
-

- $0.00 / 25KG
-
2025-03-21
- CAS:78-75-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- 1,2-Dibromopropane
-
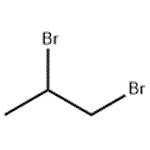
- $1.50 / 1g
-
2023-07-27
- CAS:78-75-1
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
|
| | 1,2-Dibromopropane Basic information |
| Product Name: | 1,2-Dibromopropane | | Synonyms: | Propane,1,2-dibromo-;1,2-Dibromopropane,97%;1,2-DIBROMOPROPANE;11,2-DIBROMOPROPANE;1,2-Dibromopropane,99%;(RS)-Dibromopropane;Propylene Bromide
Propylene Dibromide;1.2-DibroMopro | | CAS: | 78-75-1 | | MF: | C3H6Br2 | | MW: | 201.89 | | EINECS: | 201-139-1 | | Product Categories: | | | Mol File: | 78-75-1.mol | 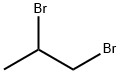 |
| | 1,2-Dibromopropane Chemical Properties |
| Melting point | -55 °C | | Boiling point | 140-142 °C(lit.) | | density | 1.937 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.519(lit.) | | Fp | 35 °C | | storage temp. | Flammables area | | solubility | organic solvents: miscible | | form | clear liquid | | color | Colorless to Light yellow to Light orange | | Water Solubility | slightly soluble | | Merck | 14,7853 | | BRN | 1718884 | | Dielectric constant | 4.2999999999999998 | | Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong bases, magnesium. | | CAS DataBase Reference | 78-75-1(CAS DataBase Reference) | | NIST Chemistry Reference | Propane, 1,2-dibromo-(78-75-1) | | EPA Substance Registry System | 1,2-Dibromopropane (78-75-1) |
| | 1,2-Dibromopropane Usage And Synthesis |
| Chemical Properties | 1,2-Dibromopropane is considered non-flammable and non-explosive liquid. | | Uses | 1,2-Dibromopropane is used in organic synthesis and solvent. It was used to study the effect of reductant concentration, reductant contact time and suspension pH on reductive dechlorination of carbon tetrachloride by soil manipulated with Fe(II) and HS-. It was used as internal standard during the determination of nitrogenous disinfection byproduct trichloronitromethane by gas chromatography mass spectrometry. | | Preparation | 1,2-Dibromopropane is synthesized from bromopropane by bromination.
First mix bromopropane and iron powder, heat to 40-50°C, slowly add bromine, and continue to reflux for 2h after adding. Then, the iron slag was filtered off, and the filtrate was washed several times with water, once with 5% sodium carbonate solution, and then with 5% sodium thiosulfate solution to remove free bromine. Dry with anhydrous calcium chloride. Fractional distillation, collecting 139-142 ℃ fraction is the finished product of 1,2-dibromopropane. | | Application | Photodissociation dynamics of 1,2-dibromopropane at 234 and 265 nm has been investigated by using velocity map ion imaging method. 1,2-Dibromopropane was used to study the effect of reductant concentration, reductant contact time and suspension pH on reductive dechlorination of carbon tetrachloride by soil manipulated with Fe(II) andHS-. It was used as internal standard during the determination of nitrogenous disinfection byproduct trichloronitromethane by gas chromatography mass spectrometry. | | General Description | 1,2-dibromopropane is a colorless liquid. Prepared from propyl bromide and Br in the presence of AlCl3 or AlBr3. (NTP, 1992) | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Halogenated aliphatic compounds, such as 1,2-Dibromopropane, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. 1,2-Dibromopropane is considered nonflammable. | | Fire Hazard | 1,2-Dibromopropane is considered non-flammable. | | Biochem/physiol Actions | 1,2-Dibromopropane induces hepatotoxicity and immunotoxicity in female BALB/c mice. |
| | 1,2-Dibromopropane Preparation Products And Raw materials |
|