2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane manufacturers
|
| | 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Basic information |
| Product Name: | 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane | | Synonyms: | 4-(tert-Butyl)naphthalene-2-boronic Acid Pinacol Ester;2-[6-[3-(1,1-dimethylethyl)-4-methoxyphenyl]-2-naphthalenyl]-4,4,5,5-tetramethyl-1,3,2-Dioxaborolane;2-[4-(1,1-Dimethylethyl)-2-naphthalenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;1,3,2-Dioxaborolane, 2-[4-(1,1-dimethylethyl)-2-naphthalenyl]-4,4,5,5-tetramethyl- | | CAS: | 2217657-10-6 | | MF: | C20H27BO2 | | MW: | 310.24 | | EINECS: | | | Product Categories: | | | Mol File: | 2217657-10-6.mol | 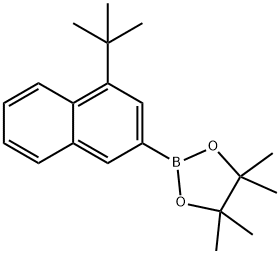 |
| | 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Chemical Properties |
| Boiling point | 423.1±24.0 °C(Predicted) | | density | 1.02±0.1 g/cm3(Predicted) |
| | 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Usage And Synthesis |
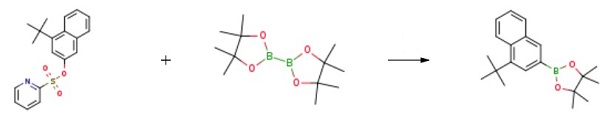
| Uses | 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane is the key intermediate for the synthesis of OLED red dopant materials has been widely concerned and studied in recent years. | | Preparation | The synthesis proceeded as follows: Successively, 600g of xylene (99%), 68.3g of intermediate B (0.2mol, 99.9%), 153.9g of biboronic acid pinacol ester (0.6mol, 99%), 1.5g of palladium acetate (0.01mol, 99%), and 16.6g of sodium acetate (0.2mol, 99%) were added. The mixture was heated to 110°C for 12 h, after which it was cooled to room temperature. The reaction mixture was then washed with water and the organic layer was dried over anhydrous sodium sulfate. Subsequently, xylene was evaporated to obtain the crude product. The crude product was recrystallized with ethyl acetate, resulting in the formation of 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane.
 |
| | 2-(4-(tert-butyl)naphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Preparation Products And Raw materials |
|