| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Tolciclate;Tolciclate
CAS:50838-36-3
Purity:98% Package:5 mg
|
| Company Name: |
CHEMICAL LAND21
|
| Tel: |
82- 2 -783 - 8063 |
| Email: |
sales21@chemicalland21.com |
| Products Intro: |
|
|
| | tolciclate Basic information |
| Product Name: | tolciclate | | Synonyms: | 1,4-Methanonaphthalene, carbamothioic acid deriv.;Carbamothioic acid, methyl(3-methylphenyl)-, O-(1,2,3,4-tetrahydro-1,4-methanonaphthalen-6-yl) ester (9CI);Fungifos;K 9147;KC 9147;Kilmicen;Tolmicen;N-(3-Methylphenyl)-N-methylthiocarbamic acid O-(1,2,3,4-tetrahydro-1,4-methanonaphthalen-6-yl) ester | | CAS: | 50838-36-3 | | MF: | C20H21NOS | | MW: | 323.45 | | EINECS: | 256-792-5 | | Product Categories: | | | Mol File: | 50838-36-3.mol | 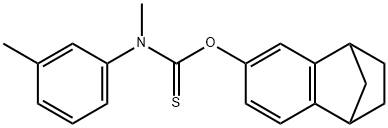 |
| | tolciclate Chemical Properties |
| Melting point | 92-94° | | Boiling point | 447.3±55.0 °C(Predicted) | | density | 1.0965 (rough estimate) | | refractive index | 1.5800 (estimate) | | pka | -0.42±0.50(Predicted) |
| Toxicity | LD50 in mice, rats, dogs (mg/kg): 4000, 6000, 5000 orally (deCarneri) |
| | tolciclate Usage And Synthesis |
| Originator | Tolmicen,Carlo Erba,Italy,1979 | | Definition | ChEBI: Tolciclate is a monothiocarbamic ester. It has a role as an antifungal drug. | | Indications | The indications for tolciclate are defined by its narrow spectrum and limited to the treatment of dermatophytoses. Because of inadequate penetration into the nail keratin, it is unlikely to be effective for the treatment of onychomycoses. On average, the efficacy of tolciclate in topical therapy of dermatomycoses is lower than that of most other antimycotics for topical treatment. | | Manufacturing Process | Thiophosgene (1.15 g, 0.01 mol) in chloroform (40 ml) was slowly treated at
room temperature with sodium 1,4-methano-1,2,3,4-tetrahydro-6-
naphthoxide (1.82 g, 0.01 mol). After 30 minutes, N-methyl-m-toluidine (2.42
g, 0.02 mol) in chloroform (40 ml) was added dropwise to the solution so
obtained at room temperature. The reaction mixture was stirred for 48 hours
at room temperature and then refluxed for 2 hours. The solvent was
evaporated, and the residue redissolved in water and extracted repeatedly
with diethyl ether. The organic phase was dried (Na2SO4) and evaporated to dryness to give, after crystallization from isopropanol, O-(1,4-methano-
1,2,3,4-tetrahydro-6-naphthyl)-N-methyl-N-(m-tolyl)-thiocarbamate (1.3 g)
melting point 92°C to 94°C. | | Therapeutic Function | Topical antimycotic | | Antimicrobial activity | In vitro, tolciclate has a narrow activity spectrum with good efficacy only towards dermatophytes. Isolates of susceptible species showing primary resistance or developing secondary resistance are rarely observed. |
| | tolciclate Preparation Products And Raw materials |
|