| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:Niobium(V) bromide (99.9%-Nb)
CAS:13478-45-0
Purity:(99.9%-Nb) Package:10g;50g
|
| Company Name: |
Shanghai Witofly Chemical Co. ,Ltd.
|
| Tel: |
021-50630626 15821189163 |
| Email: |
sales@witofly.com |
| Products Intro: |
Product Name:Niobium (V) bromide
CAS:13478-45-0
Purity:98% HPLC Package:25kg
|
|
| | NIOBIUM BROMIDE Basic information |
| Product Name: | NIOBIUM BROMIDE | | Synonyms: | NbBr5;Niobium bromide (NbBr5);niobiumbromide(nbbr5);NIOBIUM (V) BROMIDE;NIOBIUM PENTABROMIDE;NIOBIUM BROMIDE;Niobiumbromidemetalsbasismeshbrownredpowder;NIOBIUM(V) BROMIDE 98% | | CAS: | 13478-45-0 | | MF: | Br5Nb | | MW: | 492.43 | | EINECS: | 236-778-5 | | Product Categories: | metal halide | | Mol File: | 13478-45-0.mol | 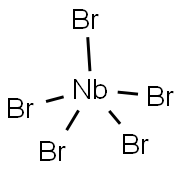 |
| | NIOBIUM BROMIDE Chemical Properties |
| Melting point | 150°C | | Boiling point | 361,6°C | | density | 4.360 | | solubility | soluble in H2O, ethanol | | form | Powder | | color | reddish-brown | | Water Solubility | soluble H2O, alcohol, ethyl bromide [KIR81] | | Sensitive | moisture sensitive | | EPA Substance Registry System | Niobium bromide (NbBr5) (13478-45-0) |
| | NIOBIUM BROMIDE Usage And Synthesis |
| Chemical Properties | yellow powder(s) or red crystals; ortho-rhomb, a=0.6127nm, b=1.2198 nm, c=1.855 nm; m.p. 265.2°C, b.p. 361.6°C; sensitive to moisture; can be formed by reacting Br2 and niobium at ~500°C [STR93] [KIR81] | | Synthesis | (1) First, the air is completely removed from the reactor. Then,
the section of the tube containing the metal is heated by a coil wound directly on the reactor, or by a tubular electric furnace o.
If Nb powder is used, then bromide formation begins at 90°C; with
solid Nb, it starts at 195°C . The nascent bromide
sublimes onto cold finger /. When larger quantities are desired,
large-diameter receiver a is attachedtotheendof the reactor. The
extremely hygroscopic bromide should be removed from the
apparatus while the latter is in a dry box.
Nb + 5/2Br2 = NbBr5.
(2) In the method of Chaigneau, the mixture of pentoxide and AlBr3,
in proportions indicated by the above equation, is sealed under
vacuum into a Pyrex glass tube (before use, the AlBr3 is purified
by vacuum sublimation). The tube is heated for 24 hours at 200°C,
and allowed to cool; the small amounts of Br 3 formed in the process
and residual AlBr3 are vacuum-sublimed at 140°C. The pure
pentahalide is then separated from the Al 3O3 by vacuum sublimation
at 240°C, yielding large crystals.
3 Nb2O5 + 10AlBr3 = 6 NbBr5 + 5A12O
(3) Nb2O5 + 3 C + 6 Br2 = 2 NbBr5 + COBr2 + 2 CO2 |
| | NIOBIUM BROMIDE Preparation Products And Raw materials |
|