|
|
| | 2,6-Dichloro-3-nitropyridine Basic information |
| Product Name: | 2,6-Dichloro-3-nitropyridine | | Synonyms: | 2,6-DICHLORO-3-NITROPYRIDINE;TIMTEC-BB SBB003614;2,6-DICHLORO-3-NITROPYRIDINE, TECH., 92%;DICHLORONITROPYRIDINE;2,6-Dichloro-3-nitro;2,6-DICHLORO-3-NITROPYRIDINE 98+%;2,6-Dichloro-3-nitropyridine ,92%;2,6-Dichloro-3-nitropyridine,90%,tech. | | CAS: | 16013-85-7 | | MF: | C5H2Cl2N2O2 | | MW: | 192.99 | | EINECS: | 240-151-1 | | Product Categories: | Building Blocks;C5;Chemical Synthesis;Halogenated Heterocycles;Heterocyclic Building Blocks;Chloropyridines;Halopyridines;C5Heterocyclic Building Blocks;Halogenated Heterocycles;Heterocyclic Building Blocks;Pyridine series;Pyridines derivates;Pyridine;pyridine derivative;Heterocycles;Pyridines;bc0001 | | Mol File: | 16013-85-7.mol | 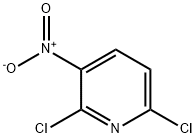 |
| | 2,6-Dichloro-3-nitropyridine Chemical Properties |
| Melting point | 55-60 °C(lit.) | | Boiling point | 295.5±35.0 °C(Predicted) | | density | 1.6791 (rough estimate) | | refractive index | 1.6100 (estimate) | | Fp | >230 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | soluble in Methanol | | form | Liquid | | pka | -5.76±0.10(Predicted) | | color | Clear colorless to light yellow | | BRN | 1619741 | | InChI | InChI=1S/C5H2Cl2N2O2/c6-4-2-1-3(9(10)11)5(7)8-4/h1-2H | | InChIKey | SHCWQWRTKPNTEM-UHFFFAOYSA-N | | SMILES | C1(Cl)=NC(Cl)=CC=C1[N+]([O-])=O | | CAS DataBase Reference | 16013-85-7(CAS DataBase Reference) |
| | 2,6-Dichloro-3-nitropyridine Usage And Synthesis |
| Chemical Properties | yellow crystalline powder or chunks | | Uses | 2,6-Dichloro-3-nitropyridine was used:
- in the synthesis of pyridyldifluoroacetates
- as starting reagent in the preparation of bicyclooxacalixhetarene
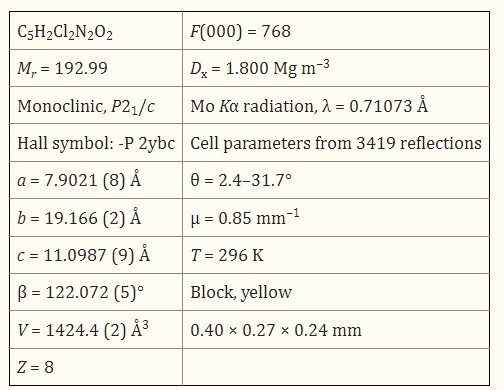
| | Definition | Nitropyridine nucleus played a pivotal role in the development of different medicinal agents and in the field of agrochemicals. It is seen from the current literature that pyridine derivatives have been developed and used as insecticidal agents. Nitrated pyridines and their derivatives are important intermediates in synthesis of heterocyclic compounds in dyes and pharmaceutical products. Fused heterocycles containing nitropyridine systems have been associated with several biological and medicinal activities including antiolytic, antiviral and anti-inflammatory profiles. | | General Description | 2,6-Dichloro-3-nitropyridine undergoes macrocyclic condensation reaction with resorcinol derivatives to yield chiral tetraoxacalix[2]arene[2]pyridines. | | Structure and conformation | The asymmetric unit of 2,6-Dichloro-3-nitropyridine consists of two crystallographically independent mol-ecules. The pyridine ring in each mol-ecule is essentially planar, with maximum deviations of 0.004 (4) and 0.007 (4)Å. Short Cl-O [3.09?(3) and 3.13 (4)Å] and Cl-Cl [3.38 (12)Å] contacts were observed. No significant inter-molecular inter-actions were observed in the crystal packing.
 |
| | 2,6-Dichloro-3-nitropyridine Preparation Products And Raw materials |
|