|
|
| | 2,4,6-Trifluorophenylboronic acid Basic information |
| Product Name: | 2,4,6-Trifluorophenylboronic acid | | Synonyms: | 2,4,6-TRIFLUOROPHENYLBORONIC ACID;RARECHEM AH PB 0121;2,4,6-Trifluoronenzeneboronic acid;2,4,6-Trifluorophenylboronic;2,4,6-TRIFLUOROBENZENEBORONIC ACID;2,4,6-Trifluorophenylboronic Acid (contains varying aMounts of Anhydride);Trifluorobenzeneboronic aci;2,4,6-trifluorophenyl(dihydroxy)borane | | CAS: | 182482-25-3 | | MF: | C6H4BF3O2 | | MW: | 175.9 | | EINECS: | 681-558-8 | | Product Categories: | FluoroCompounds;Heterocyclic Compounds;Boronic Acid;Aryl;Organoborons;Boronic Acids;Boronic Acids and Derivatives;blocks;BoronicAcids | | Mol File: | 182482-25-3.mol | 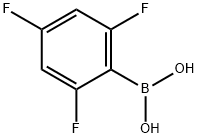 |
| | 2,4,6-Trifluorophenylboronic acid Chemical Properties |
| Melting point | 228-235 °C (lit.) | | Boiling point | 236.0±50.0 °C(Predicted) | | density | 1.44±0.1 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | soluble in Methanol | | pka | 8.26±0.58(Predicted) | | form | Crystalline Powder | | color | White to off-white | | BRN | 8549766 | | CAS DataBase Reference | 182482-25-3(CAS DataBase Reference) |
| | 2,4,6-Trifluorophenylboronic acid Usage And Synthesis |
| Chemical Properties | Off-white Cryst | | Uses | Reactant involved in:
- Synthesis of phenylboronic catechol esters
- Suzuki-Miyaura coupling reactions with aryl chlorides and unstable polyfluorophenyl
- Enantioselective borane reduction of trifluoroacetophenone
- Transmetalation
| | Uses | suzuki reaction |
| | 2,4,6-Trifluorophenylboronic acid Preparation Products And Raw materials |
|