- Selumetinib
-

- $0.00 / 25KG
-
2023-06-22
- CAS:606143-52-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Selumetinib
-

- $0.00 / 1KG
-
2023-06-19
- CAS:606143-52-6
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1000KGS
- SeluMetinib
-

- $0.00 / 1gram
-
2022-02-18
- CAS:606143-52-6
- Min. Order: 1gram
- Purity: 99%
- Supply Ability: 10kg
|
| | Selumetinib Chemical Properties |
| Melting point | >219°C (dec.) | | density | 1.69 | | storage temp. | -20° | | solubility | Soluble in DMSO (up to 50 mg/ml) or in Ethanol (up to 2 mg/ml) | | form | Beige powder. | | pka | 14.20±0.10(Predicted) | | color | White | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| | Selumetinib Usage And Synthesis |
| Indications and Usage | Selumetinib, 1 has a chemical name of 5-[(4-Bromo-2-chlorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide. It was developed by British company AstraZeneca and is used to treat advanced non-small cell lung cancer (NSCLC). It is mainly used to treat bile duct cancer, colon cancer, NSCLC, etc. Currently, Selumetinib is in stage III clinical trials for treatment of NSCLC.
| | Mechanisms of Action | Selumetinib is the first mitogenextracellular kinase (MEK1/2) inhibitor to be used in thyroid cancer clinical trials. It inhibits extracellular signal regulating kinase (ERK/2) and activates caspase to dramatically inhibit ERK1/2 phosphorylation.
| | Clinical Research | In phase II clinical trials of radioiodine-refractory papillary thyroid carcinoma, 39 patients took daily oral doses of Selumetinib (100mg bid) for 28 days; results showed that 21 patients’ conditions stabilized (54%), 11 patients’ conditions worsened (28%), 49% patients’ conditions were stable for 16 weeks, 36% patients’ conditions were stable for 24 weeks, and survival terms did not progress to 32 weeks. Negative reactions mainly consisted of rashes (59%), diarrhea (44%), and weakness (41%). Some studies found that after treating 20 patients with thyroid cancer with Selumetinib (75mg bid) for 4 weeks, Selumetinib increased the iodine uptake and retention of patients with radioiodine-refractory papillary thyroid carcinoma. In a blind and random comparative study between a Selumetinib and Docetaxel (DOC) combination treatment group and DOC and placebo treatment group for 87 mutant NSCLSC patients, survival times were 9.4 months and 5.2 months, PFS were 5.3 months and 2.1 months, RR were 37% and 0%, thus showing dramatic differences. Selumetinib’s main negative reactions include neutrophil depletion, dermatitis, and respiratory failure.
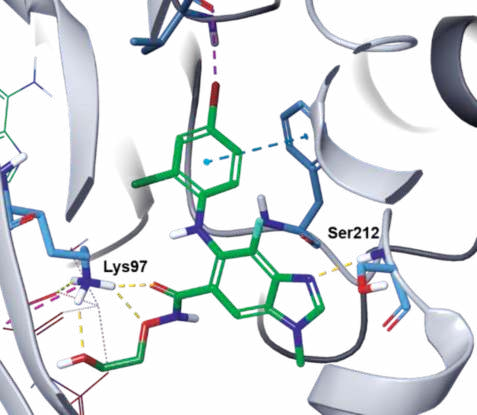
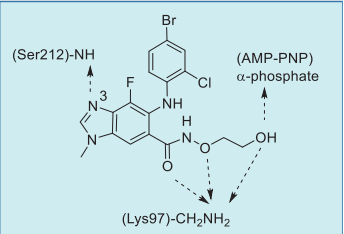
| | Binding Mode | In the co-crystal structure of selumetinib in
complex with MEK1 and AMP–PNP (Fig. 3),
selumetinib binds to a unique and specific allosteric
pocket on the N-terminal domain of MEK1, next to a
typical ATP-binding site. This binding results in a
conformational change, which prevents the RAF-induced phosphorylation, and locks MEK1/2 into a
catalytically inactive state, thereby blocking the RAS
signaling. The imine nitrogen of the
benzo[d]imidazole core hydrogen bonds to the
amide NH of Ser212, and the three oxygen atoms of
the amide side chain form three hydrogen bonds with
the primary amine of Lys97. In addition, the terminal hydroxyl group hydrogen bonds to the α-phosphoryl
oxygen of AMP–PNP (Fig. 4).
  | | Description | Selumetinib (AZD6244; ARRY-142886) is an oral MEK inhibitor. In a random�ized trial, NSCLC patients with wild-type KRAS were randomized to erlotinib
alone or combination therapy with selumetinib, while mutant KRAS patients were
randomized to selumetinib alone or combination therapy. The primary end points
were PFS for the KRAS wild-type cohort and objective response rate (ORR) for the
KRAS mutant cohort. Results were not impressive, with no PFS difference in the
KRAS wild-type arm (2.4 vs. 2.1?months) and no ORR difference in the KRAS�mutated subgroup (0% vs. 10%). A planned trial of selumetinib in combination
with the anti-PD-L1 antibody durvalumab has since been suspended (NCT03004105). | | Uses | It is a tight-binding, uncompetitive inhibitor of mitogen-activated protein kinase kinases (MEK) 1 and 2 currently in clinical development. It is useful as biomarker in human lung cancer cell. Potent MEK inhibitor. | | Definition | ChEBI: A member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-chlorophenyl)amino, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectiv
ly. It is a MEK1 and MEK2 inhibitor. | | Brand name | Koselugo | | General Description | Class: dual threonine/tyrosine kinase;
Treatment: children with NF1; Other name: AZD-6244, ARRY-142886;
Oral bioavailability = 62%;
Elimination half-life = 6.2 h;
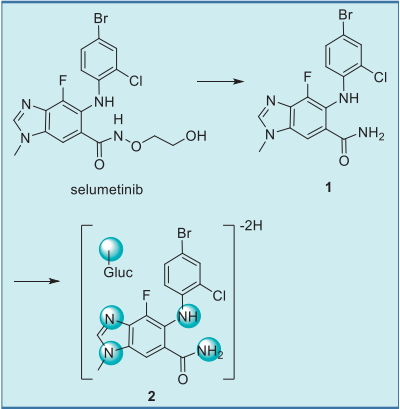
Protein binding = 97.7% | | target | MEK1 | | Metabolism | Following oral administration of radiolabeled
selumetinib, the most prominent drug-related
component in the plasma was selumetinib,
accounting for 40% of the plasma radioactivity. The
major circulating metabolite was an amide
glucuronide 2, which accounted for 22% of the
plasma radioactivity. This metabolite resulted from loss of the ethanediol moiety to give the primary
amide 1, which underwent glucuronidation and an
additional loss of 2 mass units, most likely due to
further oxidation of the N-methylbenzimidazole
moiety (Fig. 5).
 | | storage | Store at -20°C | | Dosage | Selumetinib is characterized by a moderate oral
bioavailability (62%) and a relatively short half-life
(6.2 h), and these properties contribute to twice-daily
dosing regimen (25 mg dosage). | | References | 1) Davies?et al. (2007),?AZD6244(ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamics relationship, and potential for combination in preclinical models; Mol. Cancer Ther.,?6?2209
2) Yeh?et al. (2007),?Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor; Clin. Cancer Res.,?13?1576
3) Catalanotti?et al. (2013),?Phase II trial of MEK inhibitor selumetinib(AZD6244) in patients with BRAFV600E/K-mutated melanoma; Clin. Cancer Res.,?19?2257
4) O’Neil?et al. (2011),?Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma; J. Clin. Oncol.,?29?2350
5) Khurum?et al. (2012),?A phase I dose escalation study of oral MK-2206 (allosteric Akt inhibitor) with oral selumetinib (AZD6244)(MEK 1/2 inhibitor) in patients with advanced or metastatic solid tumors; J. Clin. Oncol.,?30?e13599
6) Hainsworth?et al. (2010),?A phase II, open label, randomized study to assess the efficacy and safety of AZD6244 versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens; J. Thorac. Oncol.,?5?1630
7) Bodoky?et al. (2012),?A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy; Invest. New Drugs,?30?1216 |
| | Selumetinib Preparation Products And Raw materials |
|