- Penfluridol
-

- $47.00 / 25mg
-
2024-11-19
- CAS:26864-56-2
- Min. Order:
- Purity: 99.73%
- Supply Ability: 10g
- Penfluridol
-

- $47.00 / 25mg
-
2024-11-19
- CAS:26864-56-2
- Min. Order:
- Purity: 99.73%
- Supply Ability: 10g
- Penfluridol
-

- $120.00 / 1kg
-
2024-01-18
- CAS:26864-56-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
|
| | Penfluridol Basic information |
| Product Name: | Penfluridol | | Synonyms: | 1-[4,4-Bis(p-fluorophenyl)butyl]-4-(4-chloro-alpha,alpha,alpha-trifluoro-m-tolyl)-4-piperidinol;1-[4,4-Bis(p-flurophenyl)butyl]-4-(4-chloro-alpha,alpha,alpha-trifluoro-m-tolyl)-4-piperidinol;4-Piperidinol, 1-[4,4-bis(4-fluorophenyl)butyl]-4-[4-chloro-3-(trifluoromethyl)phenyl]-;Penfluridol, >=98%;1-[4,4-BIS(4-FLUOROPHENYL)BUTYL]-4-[4-CHLORO-3(TRIFLUOROMETHYL)PHENYL]-4-PIPERIDINOL;4-Piperidinol, 4-(4-chloro-alpha,alpha,alpha-trifluoro-m-tolyl)-1-(4,4-bis(p-fluorophenyl)butyl)-;4-piperidinol,1-(4,4-bis(4-fluorophenyl)butyl)-4-(4-chloro-3-(trifluoromethy;4-piperidinol,4-(4-chloro-alpha,alpha,alpha-trifluoro-m-tolyl)-1-(4,4-bis(p-fl | | CAS: | 26864-56-2 | | MF: | C28H27ClF5NO | | MW: | 523.97 | | EINECS: | 248-074-5 | | Product Categories: | API;API intermediates;ONCOVIN | | Mol File: | 26864-56-2.mol | 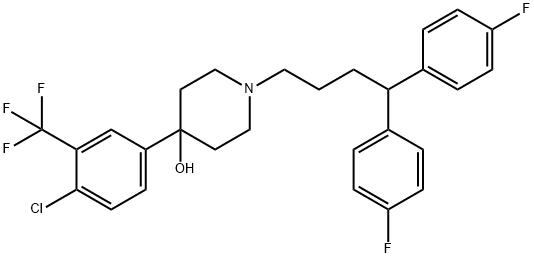 |
| | Penfluridol Chemical Properties |
| Melting point | 105-107°C | | Boiling point | 587.7±50.0 °C(Predicted) | | density | 1.2425 (estimate) | | storage temp. | 2-8°C | | solubility | H2O: insoluble | | form | powder | | pka | 13.49±0.20(Predicted) | | color | white | | Merck | 14,7087 | | CAS DataBase Reference | 26864-56-2(CAS DataBase Reference) | | NIST Chemistry Reference | Penfluridol(26864-56-2) |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | RTECS | TN6957000 | | HazardClass | 6.1 | | PackingGroup | III | | HS Code | 29333990 | | Toxicity | LD50 orally in mice (day 7): 86.8 mg/kg (Janssen) |
| | Penfluridol Usage And Synthesis |
| Originator | Semap,Janssen-Le Brun ,W. Germany ,1975 | | Uses | antineoplastic | | Uses | In terms of pharmacological action, penfluridol is similar to pimozide; however, it is sig�nificantly longer lasting, which is connected to the slow metabolism of the drug.
Penfluridol is used as a supportive therapy in ambulatory settings for patients suffering
from schizophrenia as well as patients with paranoid, psychotic, and neuroleptic condi�tions. | | Uses | Penfluridol has been used as an antipsychotic agent:
- to study its antimetastatic effect on triple-negative breast cancer cells
- to study its effects on the growth of glioblastoma cell lines,
- to study its effects on the vascular endothelial growth factor (VEGF)-induced angiogenesis in human umbilical vein endothelial cells (HUVECs)
| | Definition | ChEBI: 1-[4,4-bis(4-fluorophenyl)butyl]-4-[4-chloro-3-(trifluoromethyl)phenyl]-4-piperidinol is a diarylmethane. | | Manufacturing Process | A mixture of 24 parts of 4,4-bis(p-fluorophenyl)butyl chloride, 20.9 parts of 4(4-chloro-α,α,α-trifluoro-m-tolyl)-4-piperidinol, 13.8 parts of sodium carbonate, a few crystals of potassium iodide in 600 parts of 4-methyl-2pentanone is stirred and refluxed for 60 hours. The reaction mixture is cooled and 150 parts of water is added. The organic layer is separated, dried, filtered and evaporated. The oily residue is crystallized from diisopropylether, yielding 4-(4chloro-α,α,α-trifluoro-m-tolyl)-1-[4,4-bis(p-fiuorophenyl)butyl]-4piperidinol; melting point 106.5°C. | | Brand name | Semap (Ortho-McNeil). | | Therapeutic Function | Antipsychotic | | Biochem/physiol Actions | Penfluridol is studied in the treatment of schizophrenia, Tourette syndrome and acute psychosis. It also exhibits anticancer activity. | | Safety Profile | Poison by ingestion and intravenous routes. Experimental teratogenic and reproductive effects. A neuroleptic agent. When heated to decomposition it emits very toxic fumes of Cl-, F-, and NOx. | | Synthesis | Penfluridol, 4-(4-chloro-3-trifluoromethylphenyl)-1-[4,4-bis-(p-fluorophenyl)
butyl]-4-piperidinol (6.6.12), is synthesized implementing a Grignard reaction between
1-carbomethoxypiperidin-4-one and 4-chloro-3-trifluoromethylphenylmagnesium bromide,
giving 1-carbomethoxy-(4-chloro-3-trifluoromethylphenyl)-4-piperidinol (6.6.10). Upon
alkaline hydrolysis of the carbomethoxy group, it turns into (4-chloro-3-trifluo�romethylphenyl)-4-piperidinol (6.6.11), the alkylation of which with 1,1-bis(4-fluo�rophenyl)butyl bromide (6.6.3) gives penfluridol (6.6.12) [67¨C69]. 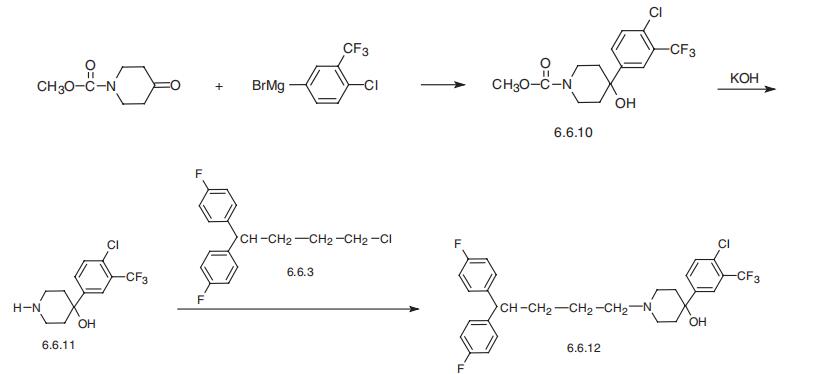
|
| | Penfluridol Preparation Products And Raw materials |
|