- Vericiguat
-

- $0.00 / 1g
-
2025-04-16
- CAS:1350653-20-1
- Min. Order: 1g
- Purity: 98%
- Supply Ability: 10kg/month
- vericiguat
-

- $0.00 / 1kg
-
2025-03-28
- CAS:1350653-20-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 100kgs
- Vericiguat
-

- $0.00 / 1g
-
2025-01-13
- CAS:1350653-20-1
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
Related articles - Synthesis of Vericiguat
- The Vericiguat synthesis was initiated with the key fluoroacrylaldehyde intermediate required to construct the fluoropyrazolop....
- Jan 12,2024
- Pharmacology of Vericiguat
- Vericiguat is a stimulator of soluble guanylate cyclase (sGC),it is used to reduce the risk of cardiovascular death and hospit....
- Jul 22,2022
- Adverse effects of vericiguat
- Vericiguat, sold under the brand name Verquvo, is a medication used to reduce the risk of cardiovascular death and heart failu....
- Feb 7,2022
|
| | vericiguat Basic information |
| Product Name: | vericiguat | | Synonyms: | vericiguat;BAY1021189;Carbamic acid, N-[4,6-diamino-2-[5-fluoro-1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-, methyl ester;Vericiguat (BAY 1021189);MK-1242;methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate (Vericiguat);Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate;Inhibitor,BAY-1021189,BAY 1021189,inhibit,Guanylate Cyclase,Vericiguat | | CAS: | 1350653-20-1 | | MF: | C19H16F2N8O2 | | MW: | 426.38 | | EINECS: | | | Product Categories: | API | | Mol File: | 1350653-20-1.mol | 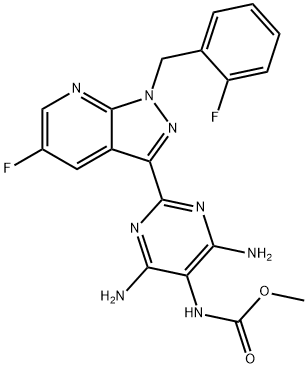 |
| | vericiguat Chemical Properties |
| Boiling point | 535.9±50.0 °C(Predicted) | | density | 1.63±0.1 g/cm3(Predicted) | | solubility | DMSO:60.0(Max Conc. mg/mL);140.7(Max Conc. mM) | | form | A solid | | pka | 10.61±0.70(Predicted) | | color | Light yellow to brown | | InChI | InChI=1S/C19H16F2N8O2/c1-31-19(30)25-14-15(22)26-17(27-16(14)23)13-11-6-10(20)7-24-18(11)29(28-13)8-9-4-2-3-5-12(9)21/h2-7H,8H2,1H3,(H,25,30)(H4,22,23,26,27) | | InChIKey | QZFHIXARHDBPBY-UHFFFAOYSA-N | | SMILES | C(OC)(=O)NC1=C(N)N=C(C2C3=CC(F)=CN=C3N(CC3=CC=CC=C3F)N=2)N=C1N |
| | vericiguat Usage And Synthesis |
| Description | Vericiguat is a novel oral soluble guanylate cyclase (sGC) stimulator.
- Soluble guanylate cyclase (sGC) is an enzyme that is activated by nitric oxide (NO). The activation initiates a signaling cascade which converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). cGMP levels increase & protein kinase G (PKG) is activated, resulting in a decrease of intracellular free Ca++ -> vascular smooth muscle cell relaxation.
- However, individuals with HF have reduced NO levels. Vericiguat, as a sGC stimulator, increases the enzymatic activity of sGC to generate cGMP independently of NO and enhances sGC sensitivity to endogenous NO.
Vericiguat is the 2nd drug in this class and follows riociguat ADEMPAS, which has been approved for treating pulmonary arterial hypertension.
SOCRATES-REDUCED was a phase II dose-finding trial of vericiguat in HF-rEF. The primary endpoint, change in NTproBNP over 12 weeks, was not statistically significant compared to placebo. A secondary exploratory analysis suggested a dose-response relationship in which higher doses of vericiguat were associated with greater reductions in NTproBNP levels. | | Uses | Vericiguat is a novel oral soluble guanylate cyclase (sGC) stimulator that enhances the cyclic guanosine monophosphate (GMP) production pathway, by directly stimulating soluble guanylate cyclase activity, as well as sensitizing soluble guanylate cyclase to endogenous NO. It was initially developed for potential to reduce mortality and morbidity associated with chronic heart failure with reduced ejection fraction. | | Uses | Vericiguat is used on targeting cyclic guanosine monophosphate in the treatment of heart failure. | | Definition | ChEBI: Vericiguat is a pyrazolopyridine that is 5-fluoro-1H-pyrazolo[3,4-b]pyridine in which the amino hydrogen at position 1 has been substituted by a 2-fluorobenzyl group and the hydrogen at position 3 has been substituted by a 4,6-diamino-5-[(methoxycarbonyl)amino]pyrimidin-2-yl group. It is a soluble guanylate cyclase stimulator which is used for treatment of chronic heart failure. It has a role as a soluble guanylate cyclase activator, a vasodilator agent and an antihypertensive agent. It is an aminopyrimidine, a pyrazolopyridine, a carbamate ester and an organofluorine compound. | | Clinical Use | Vericiguat was advanced to clinical evaluation, as an oral therapy for chronic heart failure, either with reduced ejection fraction (HFrEF), or preserved EF (HFpEF). In mid-June 2020 Merck announced that the FDA had granted priority review status to vericiguat and based on this. The FDA approved vericiguat in January 2021, to reduce the risk of cardiovascular death, heart failure re-hospitalisation, or the requirement for outpatient intravenous diuretics, in patients with symptomatic chronic heart failure and ejection fraction less than 45%. This approval was based on efficacy data arising from the Phase 3 trial NCT02861534 (a.k.a. the VICTORIA trial). | | in vivo | Vericiguat (compound 24) (oral administration; 3 mg/kg, 10 mg/kg; once daily; 21 days) maintains heart and kidney function in a model of hypertension-induced end-organ damage in L-NAME-treated renin transgenic rats. Additionally,Vericiguat-treated group substantially reduces overall mortality when compared to the control group[1]. | Animal Model: | L-NAME-treated renin transgenic rats[1] | | Dosage: | 3 mg/kg, 10 mg/kg | | Administration: | Oral administration; 3 mg/kg, 10 mg/kg; once daily; 21 days | | Result: | Resulted in a significant attenuation of blood pressure increase, however the overall rise of blood pressure increase was not halted in the 3/10 mg/kg treatment groups.Resulted a significant and dose-dependent reduction of heart hypertrophy, in both the right and left ventricle.With respect to kidney damage, Vericiguat? Led to a significant reduction in kidney injury molecule Kim-1 and osteopontin expression which are used as biomarkers for renal injury and dysfunction.Resulted in a significant and dose-dependent increase in survival rates. The rat survival rate was 70% and 90%, respectively in the 3 and 10 mg/kg qd treatment groups. In contrast, the survival rate in the placebo group was only 25% after 21 days. |
|
| | vericiguat Preparation Products And Raw materials |
|