- Labetalol hydrochloride
-

- $0.00 / 25Kg/Drum
-
2025-04-02
- CAS:32780-64-6
- Min. Order: 1KG
- Purity: 98.5%-101.0%
- Supply Ability: 500kgs
|
| | Labetalol hydrochloride Basic information |
| Product Name: | Labetalol hydrochloride | | Synonyms: | 5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)salicylamidehydrochlori;5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)-salicylamidhydroch;ah5158a;amipress;ipolab;labelol;LABETALOL HYDROCHLORIDE;SALOR-INT L479063-1EA | | CAS: | 32780-64-6 | | MF: | C19H25ClN2O3 | | MW: | 364.87 | | EINECS: | 251-211-1 | | Product Categories: | WYAMINE | | Mol File: | 32780-64-6.mol | 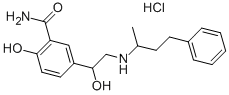 |
| | Labetalol hydrochloride Chemical Properties |
| Melting point | 187-189° | | storage temp. | 2-8°C | | solubility | H2O: soluble | | form | powder | | color | white |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36 | | WGK Germany | 3 | | RTECS | CV5376000 | | HS Code | 2924296000 | | Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 114, 120, 113, 107 i.p.; 47, 54, 60, 53 i.v.; 1450, 1800, 4550, 4000 orally (Shimpo) |
| | Labetalol hydrochloride Usage And Synthesis |
| Chemical Properties | White or almost white powder. | | Chemical Properties | Labetalol HCl is a white or off-white crystalline powder, soluble in water. Labetalol hydrochloride injection is a clear, colorless to light yellow, aqueous, sterile, isotonic solution for intravenous (IV) injection. It has a pH range of 3.0 to 4.5. Each mL contains 5 mg labetalol hydrochloride USP, 45 mg anhydrous dextrose, 0.10 mg edetate disodium; 0.80 mg of methylparaben and 0.10 mg of propylparaben as preservatives; and citric acid monohydrate and sodium hydroxide, as necessary, to bring the solution into the pH range[1]. | | Originator | Trandate,Allen and Hanburys,UK,1977 | | Uses | vasoconstrictor | | Uses | tropane alkaloid, anticholinergic drug | | Definition | ChEBI: Labetalol hydrochloride is a member of salicylamides. | | Indications | Labetalol hydrochloride is indicated for the control of blood pressure in patients with severe hypertension. Labetalol hydrochloride is also used to treat severe or mild pregnancy hypertension (PIH). | | Manufacturing Process | (a) 5-Bromoacetylsalicylamide (2.6 g), N-benzyl-N-(1-methyl-3-phenylpropyl)
amine (4.8 g) and methyl ethyl ketone (50 ml) were heated at reflux for 40
minutes. The solvent was removed and the residue was treated with benzene.
The secondary amine hydrobromide was filtered off and discarded, and the
filtrate was evaporated to dryness. The residue was treated with an excess of
ethanolic hydrogen chloride when 5-[N-benzyl-N-(1-methyl-3-phenylpropyl)-
glycyl]-salicylamide hydrochloride (1.15 g) crystallized out, MP 139°C to
141°C.
(b) 5-[N-benzyl-N-(1-methyl-3-phenylpropyl)glycyl]-salicylamide
hydrochloride (0.75 g), 10% mixture of PdO and PtO on carbon catalyst (0.1
g) and ethanol (20 ml) were shaken at room temperature and pressure with
hydrogen until uptake ceased. The catalyst was filtered off and the filtrate
evaporated to dryness. The residue was crystallized from ethanol to give 5-[1-
hydroxy-2-(1-methyl-3-phenylpropyl)aminoethyl]salicylamide hydrochloride as
a white solid (0.40 g), MP 188°C. | | Brand name | Normodyne (Schering); Trandate (Promethus). | | Therapeutic Function | Alpha-adrenergic blocker, Beta-adrenergic blocker | | Biological Activity | labetalol hcl is a selective antagonist for the α1-adrenergic receptor and a non-selective antagonist for the β-adrenergic receptor. labetalol hcl has been used for the treatment of high blood pressure. labetalol hcl competitively targets the α1-adrenergic receptors expressed in vascular smooth muscle, thus inhibiting adrenergic stimulation on endothelial cells and vasoconstriction in peripheral blood vessels. labetalol hcl also blocks the β-adrenergic receptors in bronchial and vascular smooth muscle to counteract adrenergic stimulation. ultimately, labetalol hcl causes decreases in resting and exercise heart rates, cardiac output, as well as in both systolic and diastolic blood pressure, exherting vasodilation, as well as negative chronotropic and inotropic cardiac effects.1. national center for biotechnology information (2021). pubchem compound summary for cid 3869, labetalol. retrieved august 21, 2021.2. dage rc, hsieh cp. direct vasodilatation by labetalol in anaesthetized dogs. british journal of pharmacology, 1980, 70(2): 287-293. | | References | [1] Labetalol Hydrochloride[J]. Definitions, 2020. DOI:10.1002/9780470909799.ch11. |
| | Labetalol hydrochloride Preparation Products And Raw materials |
|