| Company Name: |
Beijing Comparison Pharmaceutical Technology Co., Ltd
|
| Tel: |
010-010-52878169 15801002657 |
| Email: |
sales@bjcomparison.com |
| Products Intro: |
Product Name:(S,E)-6-(2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)vinyl)-5,6- dihydro-2H-pyran-2-one
CAS:148516-15-8
Purity:>=98% Package:100mg
|
| Company Name: |
ChemStrong Scientific Co.,Ltd
|
| Tel: |
0755-0755-66853366 13670046396 |
| Email: |
sales@chem-strong.com |
| Products Intro: |
Product Name:Pitavastatin Impurity 50
CAS:148516-15-8
Purity:95% HPLC Package:10mg;25mg;50mg;100mg
|
|
| | Pitavastatin Impurity 29 Basic information |
| Product Name: | Pitavastatin Impurity 29 | | Synonyms: | (S,E)-6-(2-(2-cyclopropyl-4-;2H-Pyran-2-one, 6-[(1E)-2-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-5,6-dihydro-, (6S)-;(6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-5,6-dihydro-2H-pyran-2-one;(S,E)-6-(2-(-Cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)vinyl)-5,6-dihydro-2H-pyran-2-one;(S,E)-6-(2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)vinyl)-5,6- dihydro-2H-pyran-2-one;(S,E)-6-(2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)vinyl)-5,6-dihydro-2H-pyran-2-one? (Pitavastatin Impurity) | | CAS: | 148516-15-8 | | MF: | C25H20FNO2 | | MW: | 385.43 | | EINECS: | | | Product Categories: | | | Mol File: | 148516-15-8.mol | 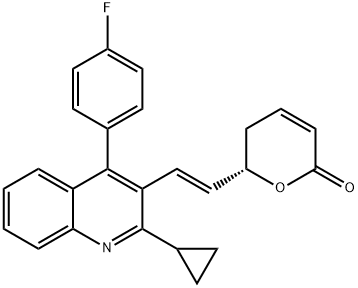 |
| | Pitavastatin Impurity 29 Chemical Properties |
| Boiling point | 590.0±50.0 °C(Predicted) | | density | 1.328±0.06 g/cm3(Predicted) | | pka | 4.47±0.50(Predicted) |
| | Pitavastatin Impurity 29 Usage And Synthesis |
| Uses | A Phenylquinoline derivative. It is an impurity in the production of Pitavastatin, which is a competitive inhibitor of HMG-CoA reductase. Antilipemic. |
| | Pitavastatin Impurity 29 Preparation Products And Raw materials |
|