- Neflamapimod
-

- $43.00 / 5mg
-
2024-11-17
- CAS:209410-46-8
- Min. Order:
- Purity: ≥95%
- Supply Ability: 10g
|
| Product Name: | VX-745 | | Synonyms: | 6H-PYRIMIDO[1,6-B]PYRIDAZIN-6-ONE, 5-(2,6-DICHLOROPHENYL)-2-[(2,4-DIFLUOROPHENYL)THIO]- (9CI);6H-PYRIMIDO[1,6-B]PYRIDAZIN-6-ONE, 5-(2,6-DICHLOROPHENYL)-2-[(2,4-DIFLUOROPHENYL)THIO]-;5-(2,6-Dichlorophenyl)-2-(2,4-difluorophenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one;5-(2,6-Dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyridazino[6,1-f]pyrimidin-6-one;5-(2,6-Dichlorophenyl)-2-(2,4-difluorophenylsulfanyl)-6H-pyriMido[3,4-b]pyridazin-6-one;VX-745(VX 745);5-(2,6-Dichloro-phenyl)-2-(2,4-difluoro-phenylsulfanyl)-1,7,8a-triaza-naphthalen-6-one;5-(2,6-Dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyridazino[6,1-f]pyrimidin-6-one VX-745 | | CAS: | 209410-46-8 | | MF: | C19H9Cl2F2N3OS | | MW: | 436.26 | | EINECS: | | | Product Categories: | MAPK;Aromatics;Heterocycles;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds | | Mol File: | 209410-46-8.mol | 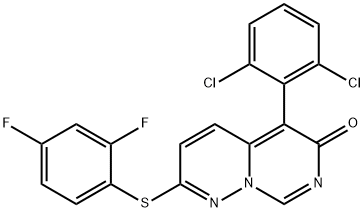 |
| | VX-745 Chemical Properties |
| Melting point | 261-264 °C | | Boiling point | 578.9±60.0 °C(Predicted) | | density | 1.55±0.1 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | ≥21.8 mg/mL in DMSO; insoluble in H2O; ≥2.1 mg/mL in EtOH with gentle warming and ultrasonic | | form | powder | | pka | -0.97±0.40(Predicted) | | color | white to beige |
| | VX-745 Usage And Synthesis |
| Description | VX-745 is an inhibitor of p38α MAPK (IC50 = 9 nM). It is selective for p38α over p38β MAPK (Ki = 220 nM) as well as ERK, JNK, and a panel of 50 kinases when used at a concentration of 2 μM. VX-745 inhibits LPS-induced production of IL-1β and TNF-α in isolated human peripheral blood mononuclear cells (PBMCs; IC50s = 45 and 51 nM, respectively). It reduces disease severity in a type II collagen-induced mouse model of arthritis when administered at a dose of 10 mg/kg. | | Chemical Properties | Pale Yellow Solid | | Uses | VX 745 is a potent and selective inhibitor of p38α mitogen-activated protein (MAP) kinase. VX 745 is a potential anti-inflammatory agents. Studies suggest that VX 745 may be useful in the treatment of Werner syndrome. | | Definition | ChEBI: A member of the class of pyrimidopyridazines that is 6H-pyrimido[1,6-b]pyridazin-6-one substituted at positions 2 and 5 by (2,4-difluorophenyl)sulfanyl and 2,6-dichlorophenyl groups respectively | | Biochem/physiol Actions | VX-745 is a selective blood-brain barrier penetrant inhibitor of p38 MAPKα. VX-745 showed 20-fold selectivity for p38α over p38β and a Ki value of 220 nM. VX-745 has been shown to reduce inflammation in an arthritic (AIA) mouse model and to improve performance in aged rats. | | storage | -20°C | | references | [1]. bagley, m.c., et al., microwave-assisted ullmann c-s bond formation: synthesis of the p38alpha mapk clinical candidate vx-745. j org chem, 2009. 74(21): p. 8336-42.
[2]. dent, p., et al., mapk pathways in radiation responses. oncogene, 2003. 22(37): p. 5885-96.
[3]. dominguez, c., d.a. powers, and n. tamayo, p38 map kinase inhibitors: many are made, but few are chosen. curr opin drug discov devel, 2005. 8(4): p. 421-30.
[4]. duffy, j.p., et al., the discovery of vx-745: a novel and selective p38alpha kinase inhibitor. acs med chem lett, 2011. 2(10): p. 758-63.
[5]. bagley, m.c., et al., gram-scale synthesis of the p38alpha mapk-inhibitor vx-745 for preclinical studies into werner syndrome. future med chem, 2010. 2(9): p. 1417-27. |
| | VX-745 Preparation Products And Raw materials |
|