- Imipenem
-

- $0.00 / 25kg
-
2025-04-14
- CAS:74431-23-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- Imipenem
-

- $0.00 / 1kg
-
2025-04-02
- CAS:74431-23-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg
- Imipenem
-

- $40.00 / 1kg
-
2025-03-07
- CAS:74431-23-5
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
Related articles - What is Imipenem?
- Imipenem (N-formimidoylthienamycin monohydrate) is a crystalline derivative of thienamycin, which is produced by Streptomyces ....
- Feb 19,2020
|
| Product Name: | Imipenem | | Synonyms: | Imipenem hydrate;Imipenem Monohydrate (100 mg);Crude Grade of Imipenem;(5R,6S)-6-[(1R)-1-Hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}thio)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate, Primaxin monohydrate, Tienam monohydrate;(6 R,7 R)-7-AMIMO-8-OXO-3-(1-PROPENYL)-5-THIA-1-AZABICYCLO[4.2.0]OCT-2-ENE-2-CARBOXYLIC ACID Monohydrate;(5R,6S)-3-((2-Formimidamidoethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid hydrate;Primaxin monohydrate;Tienam monohydrate | | CAS: | 74431-23-5 | | MF: | C12H19N3O5S | | MW: | 317.36 | | EINECS: | 680-398-6 | | Product Categories: | ROXIT;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 74431-23-5.mol |  |
| | Imipenem Chemical Properties |
| Melting point | 193-198°C | | Boiling point | 567℃ | | RTECS | CL5446516 | | Fp | >110°(230°F) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | H2O: >5mg/mL | | pka | pKa 3.2/9.9(H2O,t = 25) (Uncertain) | | form | powder | | color | white to beige | | optical activity | [α]/D +73 to +84°, c =0.5 in water | | Water Solubility | Soluble in water at 5mg/ml | | CAS DataBase Reference | 74431-23-5(CAS DataBase Reference) |
| WGK Germany | 3 | | HS Code | 2941906000 |
| | Imipenem Usage And Synthesis |
| Chemical Properties | Imipenem (N-formimidoylthienamycin monohydrate) is a crystalline derivative of thienamycin, which is produced by Streptomyces cattleya. Its chemical name is (5 R,6S)-3-[[2-(formimidoylamino)ethyl]thio]-6-[( R)-1-hydroxyethyl ]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate. It is an off-white, nonhygroscopic crystalline compound with a molecular weight of 317.37. It is sparingly soluble in water and slightly soluble in methanol.
| | Discovery | Imipenem is an intravenous β-lactam antibiotic discovered by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s. Imipenem was patented in 1975 and approved for medical use in 1985.
Imipenem has a broad spectrum of activity against aerobic and anaerobic, Gram-positive and Gram-negative bacteria. It is particularly important for its activity against Pseudomonas aeruginosa and the Enterococcus species. It is not active against MRSA, however.
| | Broad-spectrum antibiotic | Imipenem is a semisynthetic thienamycin that has a wide spectrum of antibacterial activity against gram-negative and gram-positive aerobic and anaerobic bacteria, including many multiresistant strains.It is stable to many beta-lactamases. Similar compounds include meropenem, known for having greater activity against Gram negative bacteria, and the newer ertapenem which exhibits a longer half-life due to increased binding to plasma proteins.
Imipenem acts as an antimicrobial through the inhibition of cell wall synthesis of various gram-positive and gram-negative bacteria.This inhibition of cell wall synthesis in gram-negative bateria is attained by binding to penicillin-binding proteins (PBPs).
| | Chemical Properties | Off-White Solid | | Uses | antidiabetic | | Uses | antiulcer | | Uses | An extremely broad-spectrum semi-synthetic antibiotic | | Uses | Imipenem has a broad spectrum of antimicrobial action, which includes most clinically
significant microorganisms: Gram-positive, Gram-negative, aerobic, and anaerobic. It is
resistant with respect to most beta-lactamases of Gram-positive and Gram-negative bacte�ria. It is used for bacterial infections of the lower respiratory tract, infections of the urinary
and sexual tracts, infections of bones, joints, skin, soft tissues, intraabdominal and gyne�cological infections, bacterial septicemia, and endocarditis.
Imipenem undergoes enzymatic inactivation in the kidneys. In order to avoid this problem,
it is used in a 1:1 ratio in combination with cilastatin—the sodium salt of [R-[R,S-(Z)]]-7-[(2-
amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)aminocarbonyl-2-heptenoic acid
(32.1.3.6), which inhibits metabolisms of imipenem in the kidneys. This combination of two
compounds is also used in medicine under the name primaxin. | | Definition | ChEBI: Imipenem hydrate is a member of carbapenems. It contains an imipenem. | | General Description | Chemical structure: ?-lactam | | Biochem/physiol Actions | Imipenem is found effective against gram positive and negative aerobes and anaerobes. Imipenem is often combined with cilastatin, to inhibit its metabolism in kidney. | | Synthesis | Imipenem, [5R-[5|á,6|á(R)]]-6-(1-hydroxyethyl)-3-[[2-[(iminomethyl)amino]
ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2-en-2-carboxylic acid (32.1.3.1), is the only car�bapenem presently used in clinics. It is synthesized from thienamycin isolated from
Streptomyces cattleya by reacting it with the methyl formimidate. 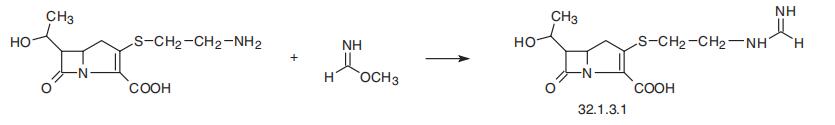
Unlike penicillins and cephalosporins, which have a side aminoacyl group joined to the beta�lactam ring, imipenem has a |á-hydroxyethyl side chain. Significant resistance to hydrolysis
by beta-lactamases is observed in this compound, evidently thanks to the trans-configuration
of the side chain, while the side chain of penicillins and cephalosporins have a cis configuration. |
| | Imipenem Preparation Products And Raw materials |
|