|
|
| | 2,6-Dichlorophenyl isocyanate Basic information |
| Product Name: | 2,6-Dichlorophenyl isocyanate | | Synonyms: | 1,3-dichloro-2-isocyanatobenzene;1,3-dichloro-2-phenylisocyanate;2,6-DICHLOROPHENYL ISOCYANATE;Isocyanic acid-2,6-dichlorophenyl ester;2,6-DICHLOROPHENYL ISOCYANATE 98%;2,6-Dichlorophenyl;2,6-Dichlorophenyl isocyanate, 98% 5GR;2,6-Dichlorophenyl isocyanate,98% | | CAS: | 39920-37-1 | | MF: | C7H3Cl2NO | | MW: | 188.01 | | EINECS: | 254-699-4 | | Product Categories: | Isocyanates;Nitrogen Compounds;Organic Building Blocks | | Mol File: | 39920-37-1.mol | 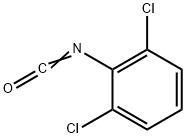 |
| | 2,6-Dichlorophenyl isocyanate Chemical Properties |
| Melting point | 43-45°C | | Boiling point | 101°C 5mm | | density | 1,015 g/cm3 | | refractive index | 1.5500 (estimate) | | Fp | 76 °C | | solubility | soluble in Toluene | | form | powder to lump to clear liquid | | color | White or Colorless to Almost white or Almost colorless | | Water Solubility | decomposes | | Sensitive | Moisture Sensitive | | BRN | 608323 | | CAS DataBase Reference | 39920-37-1(CAS DataBase Reference) | | EPA Substance Registry System | Benzene, 1,3-dichloro-2-isocyanato- (39920-37-1) |
| | 2,6-Dichlorophenyl isocyanate Usage And Synthesis |
| Chemical Properties | The dichlorophenyl isocyanates are combustible, crystalline (sugar or sand-like) solids. In general, they
are white to yellow in color, but the 1,4-dichloro-2-phenyl
isomer is white to light green. Their flash points are
generally .113�C but that of the 1,3-dichloro-2-phenyl isomer is reported as 77�C. These chemicals are insoluble in
water, and some may be reactive. 1,2-dichloro-4-isomer
(CAS 102-36-3) is the isomer of regulatory focus | | Chemical Properties | white to yellow crystalline mass | | Uses | 2,6-Dichlorophenyl isocyanate has been used in the preparation of pyridine-urea analog. | | Potential Exposure | Those materials used as chemical
intermediates | | Shipping | UN2250 Dichlorophenyl isocyanates, Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | | Incompatibilities | May form explosive mixture with air.
Isocyanates are highly flammable and reactive with many
compounds, even with themselves. Incompatible with
oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Reaction with moist air, water or
alcohols may form amines and insoluble polyureas and
react exothermically, releasing toxic, corrosive or flammable gases, including carbon dioxide; and, at the same time,
may generate a violent release of heat increasing theconcentration of fumes in the air. Incompatible with
amines, aldehydes, alkali metals, ammonia, carboxylic
acids, caprolactum, alkaline materials, glycols, ketones,
mercaptans, hydrides, organotin catalysts, phenols, strong
acids, strong bases, strong reducing agents such as
hydrides, urethanes, and ureas. Elevated temperatures or
contact with acids, bases, tertiary amines, and acylchlorides may cause explosive polymerization. Contact
with metals may evolve flammable hydrogen gas. Attacks
some plastics, rubber, and coatings. | | Waste Disposal | Combustion in an incinerator
equipped with afterburner and fume scrubber |
| | 2,6-Dichlorophenyl isocyanate Preparation Products And Raw materials |
|