| Company Name: |
Henan Weituxi Chemical Technology Co., Ltd.
|
| Tel: |
0371-63284658 18135795563 |
| Email: |
2885349392@qq.com |
| Products Intro: |
Product Name:methylvinylnitrosamine
CAS:4549-40-0
Purity:87% Package:1g 5g 10g 25g 100g 500g;质量保证;可分装;详询:18135795563(微信同号);QQ:2885349392
|
| Company Name: |
Qi Qi hang Biotechnology Co., Ltd.
|
| Tel: |
18151111370 18151111370 |
| Email: |
1046215251@qq.com |
| Products Intro: |
Product Name:Methylvinylnitrosamine
CAS:4549-40-0
Purity:98 Package:100g Remarks:QQ1046215251
|
|
| | Methylvinylnitrosamine Basic information |
| Product Name: | Methylvinylnitrosamine | | Synonyms: | N-nitroso-N-methylvinylamine;N-METHYL-N-VINYLNITROSAMINE;Vinylamine,N-methyl-N-nitroso-;Methylvinylnitrosoamine;N-Methyl-N-nitrosovinylamine;N-ethenyl-N-methylnitrous amide;N-ethenyl-N-methyl-nitrous amide;N-methyl-N-vinyl-nitrous amide | | CAS: | 4549-40-0 | | MF: | C3H6N2O | | MW: | 86.09 | | EINECS: | | | Product Categories: | | | Mol File: | 4549-40-0.mol | 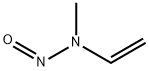 |
| | Methylvinylnitrosamine Chemical Properties |
| Boiling point | 158.75°C (rough estimate) | | density | 1.1530 (rough estimate) | | refractive index | 1.5110 (estimate) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Oil | | pka | -5.74±0.70(Predicted) | | color | Yellow | | Stability: | Light Sensitive, Volatile | | IARC | 2B (Vol. 17, Sup 7) 1987 | | EPA Substance Registry System | N-Nitrosomethylvinylamine (4549-40-0) |
| | Methylvinylnitrosamine Usage And Synthesis |
| Chemical Properties | N-Nitrosomethylvinylamine is a yellow liquid. | | Uses | N-Methyl-N-nitroso-ethenamine is used as research reagent for base induced fragmentation to give aldehydes or ketones and smaller alkylnitrosamine | | Uses | N-nitrosomethylvinylamine is used as a research chemical; no other uses were identified (IARC 1978, HSDB 2009). | | Production Methods | NMVA has not been produced commercially and is currently
produced in limited quantities for research purposes. There
are no reports that it occurs in the environment and exposure
is likely limited to researchers using NMVA. | | Definition | ChEBI: N-Nitrosomethylvinylamine is a nitroso compound. | | General Description | Very volatile yellow liquid. | | Air & Water Reactions | Water soluble but relatively unstable in water . | | Health Hazard | ACUTE/CHRONIC HAZARDS: Methylvinylnitrosamine is highly toxic by ingestion and inhalation. When heated to decomposition, Methylvinylnitrosamine emits toxic fumes of NOx. | | Fire Hazard | Flash point data for Methylvinylnitrosamine are not available; however Methylvinylnitrosamine is probably combustible. | | Safety Profile | Confirmed carcinogen
with experimental tumorigenic data. Poison
by ingestion and inhalation. When heated to
decomposition it emits toxic fumes of NOx.
See also N-NITROSO COMPOUNDS and
AMINES. | | Potential Exposure | This chemical is not manufactured but
occurs as a chemical reaction byproduct found in the dye,
automotive, rubber, and leather industries. | | Carcinogenicity | N-Nitrosomethylvinylamine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | Shipping | UN3082 Environmentally hazardous substances,
liquid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous
hazardous material, Technical Name Required | | Incompatibilities | Light sensitive. Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause
fires or explosions. Keep away from alkaline materials strong bases, strong acids, oxoacids, epoxides. A nitrated
amine. Amines are combustible. Azo, diazo, azido compounds
can detonate. This applies in particular to organic
azides that have been sensitized by the addition of metal
salts or strong acids. Toxic gases are formed by mixing
materials of this class with acids, aldehydes, amides, carbamates,
cyanides, inorganic fluorides, halogenated organics,
isocyanates, ketones, metals, nitrides, peroxides, phenols,
epoxides, acyl halides, and strong oxidizing or reducing
agents. Flammable gases are formed by mixing materials in
this group with alkali metals. Explosive combination can
occur with strong oxidizing agents, metal salts, peroxides,
and sulfides. | | Waste Disposal | Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal. |
| | Methylvinylnitrosamine Preparation Products And Raw materials |
|