| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Azapropazone;Azapropazone
CAS:13539-59-8
Purity:98% Package:5 mg
|
|
| | azapropazone Basic information |
| Product Name: | azapropazone | | Synonyms: | AHR-3018;Mi-85;NSC-102824;Rheumox;AZAPROPAZONE IMPURITY C BP(CRM STANDARD);AZAPROPAZONE IMPURITY B BP STANDARD(CRM STANDARD);AZAPROPAZONE BP STANDARD(CRM STANDARD);AZAPROPAZONE IMPURITY STANDARD BP(CRM STANDARD) | | CAS: | 13539-59-8 | | MF: | C16H20N4O2 | | MW: | 300.36 | | EINECS: | 2369138 | | Product Categories: | | | Mol File: | 13539-59-8.mol | 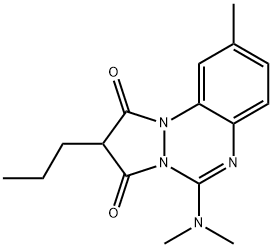 |
| | azapropazone Chemical Properties |
| Melting point | 228° | | Boiling point | 441.59°C (rough estimate) | | density | 1.0975 (rough estimate) | | refractive index | 1.6000 (estimate) | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 8.66±0.20(Predicted) | | Water Solubility | 147.2mg/L(35 ºC) |
| Toxicity | LD50 orl-rat: 1800 mg/kg OYYAA2 15,41,78 |
| | azapropazone Usage And Synthesis |
| Description | Apazone is a complex pyrazolidinedione
used at 600 ~ 1200 mg/d to achieve an anti-inflammatory effect comparable to phenylbutazone. It is tightly bound to plasma protein and
may displace anticoagulants and hypoglycemic
agents. Its plasma half-life is 8 ~ 12 h. It is well
tolerated with only a low incidence of gastric
disturbances. As with all pyrazolone derivatives,
the potential for bone marrow suppression exists
and careful monitoring of the patient's hematologic state is recommended. | | Chemical Properties | Nearly colorless, crystalline solid. | | Originator | Prolixan,Siegfried,W. Germany,1970 | | Uses | An anti-inflammatory, analgesic, and antipyretic. It
is also useful in the treatment of gout due to its uricosuric
actions. | | Uses | Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory. | | Definition | ChEBI: A member of the class of benzotriazines that is 1,2-dihydro-1,2,4-benzotriazine bearing a dimethylamino substitutent at position 3 and a methyl substituent at position 7 and in which the nitrogens at positions 1 and 2 are both acylated by a carboxy group o
propylmalonic acid. | | Manufacturing Process | The following describes two alternatives for the synthesis of the closely
related butyl analog.
Alternative (a): In a three-neck flask with descending condenser to 3.8 grams
of 3-dimethylamino-(1,2-dihydro-1,2,4-benzotriazine) are added 0.52 gram
metallic sodium, dissolved in a small volume of absolute alcohol, 4.5 g of
diethylbutylmalonate (diethylpropylmalonate for Apazone) and 15 ml of
xylene, in a nitrogen atmosphere. The mixture is heated for 2 hours to 70°C,
then for 3 hours to 110-130°C and for one more hour to 150°C, slowly
distilling off the alcohol and most of the xylene. To the resulting light brown
colored mass are added 200 ml of water. The resulting solution is extracted
twice with ether or benzene and afterwards acidified with HCl. Yield 3.6 g of
1,2-butylmalonyl-3-dimethylamino-(1,2-dihydro-1,2,4-benzotriazine). After
crystallization from alcohol the melting point is 189-190°C.
Alternative (b): 3-Dimethylamino-1,2,4-benzotriazine oxide is shaken in the
presence of Raney nickel in 15 volume parts of an alcohol-acetic acid (9:1)
mixture in a hydrogen atmosphere. The mixture absorbs 2 mols hydrogen per
1 mol starting material. Hydrogenation can also be effected using a palladium
catalyst with a suitable solvent. After reduction it is filtered on a Buchnerfunnel
through a Hyflow-layer and the solvent is evaporated in vacuum under
nitrogen. The residue is dissolved in 20 parts of water-free dioxane and
treated at 60°C with the calculated amount of butylmalonyl chloride (propyl
malonyl chloride for Apazone) (1 mol/mol) and triethylamine (2 mol/mol). The
separated triethylamine hydrochloride is filtered, the dioxane-solution is
evaporated under vacuum to dryness, and the residue is dissolved in 7
volume parts of boiling acetic acid. After cooling, the product separates in
lightly yellowish crystals. They are dissolved in the calculated amount of 0.25
N NaOH, treated with a small amount of carbon and precipitated with HCl.
Melting point of the purified product is 187°C. Yield: approximately 60% of
the theoretical amount. | | Brand name | Ahr 3018;Azapren;Cinnamin;Cinnopropazone;Dolo-prolixan;Pentosol;Prodisan;Prolixana;Sinnamin;Tolyprina;Xani. | | Therapeutic Function | Antiarthritic | | World Health Organization (WHO) | Azapropazone, which has anti-inflammatory, analgesic and
antipyretic activity, was introduced in 1970 for the treatment of rheumatic
disorders. Although sometimes classified as a pyrazolone derivative, the
relationship with this group of compounds has been disputed and classification as
a benzotriazine derivative might be preferable. Although, to date, it has not been
associated with blood dyscrasias, some regulatory authorities have applied the
same rigorous restrictions to its indications as they have applied to pyrazolone
derivatives. The World Health Organization was informed that as of December 1987
azapropazone was available in some 27 countries. | | Hazard | Nausea, vomiting, abdominal pain, gastric ulcers; moderately toxic. | | Safety Profile | Moderately toxic by ingestion,intraperitoneal and intravenous routes. When heated todecomposition it emits toxic fumes of NOx. | | Synthesis | Synthesis: a mixture of 1,2-dihydro-3-dimethylamino-7-methylbenzotriazine, diethyl
propylmalonate, and sodium ethoxide in xylene is heated over a period of time to 150 ?C,
cooled, and acidified to give the desired product.
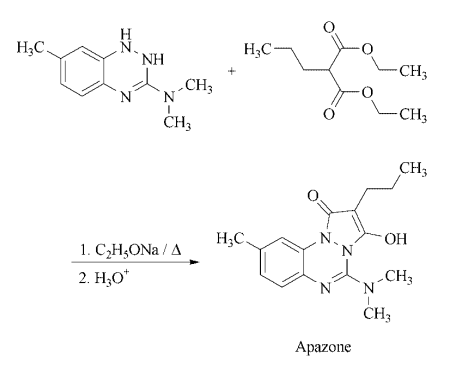 |
| | azapropazone Preparation Products And Raw materials |
|