| Company Name: |
Shaanxi Dideu Newmaterial Co., Ltd.
|
| Tel: |
029-87576359 15353716720 |
| Email: |
1039@dideu.com |
| Products Intro: |
Product Name:Vanadium, dichlorooxo-
CAS:10213-09-9
Purity:98% Package:25kg
|
| Company Name: |
Hangzhou Ocean Chemical Co., Ltd
|
| Tel: |
0571-88025872 13588138709 |
| Email: |
sales@hzoceanchem.com |
| Products Intro: |
Product Name:Vanadium(Ⅳ) oxysulfate dihydrate
CAS:10213-09-9
|
|
| | vanadium dichloride oxide Basic information |
| Product Name: | vanadium dichloride oxide | | Synonyms: | Vanadium(IV) oxychloride;Vanadium(IV) oxidedichloride;Vanadium(IV) dichlorideoxide;Vanadium(IV) dichloride oxide;Dichlorooxovanadium(IV);dichlorooxo-Vanadium;dichlorooxovanadium;VANADYLOXYDICHLORIDE | | CAS: | 10213-09-9 | | MF: | Cl2OV+ | | MW: | 137.8469 | | EINECS: | 233-517-7 | | Product Categories: | | | Mol File: | 10213-09-9.mol |  |
| | vanadium dichloride oxide Chemical Properties |
| density | 2.88 | | solubility | reacts with H2O; soluble in ethanol | | form | green hygroscopic crystals | | color | green hygroscopic crystals, crystalline | | Water Solubility | slowly decomposed in water; soluble absolute alcohol, glacial acetic acid [MER06] | | EPA Substance Registry System | Vanadium, dichlorooxo- (10213-09-9) |
| RIDADR | 2923 | | HazardClass | 8 | | PackingGroup | III |
| | vanadium dichloride oxide Usage And Synthesis |
| Chemical Properties | Green, deliquescent crystal. Usual technical
product is a dark-green, syrupymass, 76–82%
pure, or a solution. Slowly decomposed by water;
soluble in water, alcohol, and acetic acid; may react
violently with potassium. | | Uses | Vanadium Dichloride Oxide has been used as mordant in printing fabrics. | | Preparation | A thoroughly ground mixture of 3.6 g. of dry V2O5 and 9.4 g. of VCl3 is placed at the closed end of a l-m.-longtube, and 0.9 ml. of VOCl3 is then added. The upper part of the tube must be free of traces of these substances. The tube, filled with air, is melt-sealed, and is covered along its entire length with a sheet-metal jacket; its lower third, in a slightly inclined position, is then heated to about 600℃ with a tubular electric furnace. The sheet-metal jacket provides a temperature gradient along which the product VOCl2 sublimes out of the hot reaction zone. This procedure requires at least 4-5 days. However, the yield can be increased by longer heating time. Green needlelike crystals of VOCl2 are deposited in the cold part of the tube. The tube is opened at a suitable spot; the product is suspended in petroleum ether, ethyl ether or CCl4 to dissolve some adhering VOCl3, and then suction-filtered on a coarse fritted-glass filter. The relatively coarse filter separates the VOCl2 crystals from traces of finely divided hydrolysis products. The VOCl2 is freed of adhering solvent and stored under anhydrous conditions.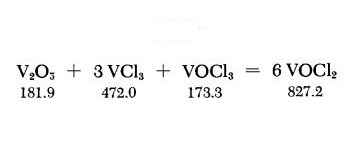 | | Hazard | Strong irritant to tissue. |
| | vanadium dichloride oxide Preparation Products And Raw materials |
|