| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Email: |
inquiries@lgmpharma.com |
| Products Intro: |
Product Name:Atevirdine
CAS:136816-75-6
Purity:Typically NLT 98%
|
| Company Name: |
Hangzhou Sage Chemical Co., Ltd.
|
| Tel: |
+86057186818502 13588463833 |
| Email: |
info@sagechem.com |
| Products Intro: |
Product Name:Methanone, [4-[3-(ethylaMino)-2-pyridinyl]-1-piperazinyl](5-Methoxy-1H-indol-2-yl)-
CAS:136816-75-6
Purity:98% Package:1g;5g;100g;Bulk
|
| Company Name: |
Shanghai Chaolan Chemical Technology Center
|
| Tel: |
QQ:65489617 15618227136 |
| Email: |
info@SuperLan-chem.com |
| Products Intro: |
Product Name:Atevirdine
CAS:136816-75-6
Purity:98% Package:5MG;10MG;50MG;100MG,1G,5G,100G
|
|
| | atevirdine Basic information |
| Product Name: | atevirdine | | Synonyms: | atevirdine;Methanone, [4-[3-(ethylaMino)-2-pyridinyl]-1-piperazinyl](5-Methoxy-1H-indol-2-yl)-;[4-[3-(ethylamino)pyridin-2-yl]piperazin-1-yl]-(5-methoxy-1H-indol-2-yl)methanone | | CAS: | 136816-75-6 | | MF: | C21H25N5O2 | | MW: | 379.46 | | EINECS: | | | Product Categories: | | | Mol File: | 136816-75-6.mol | 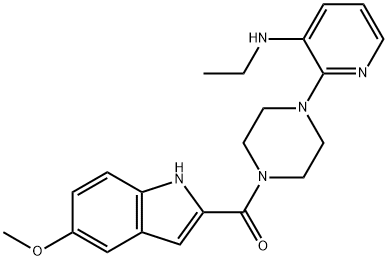 |
| | atevirdine Chemical Properties |
| | atevirdine Usage And Synthesis |
| Originator | Atevirdine,Upjohn | | Uses | Antiviral. | | Manufacturing Process | 1-[5-Methoxyindolyl-2-carbonyl]-4-[3-(ethylamino)-2-pyridinyl]piperazine:
1,1'-Carbonyldiimidazole (0.55 g) is added to a 20°-25°C solution of 5-
methoxyindole-2-carboxylic acid (0.59 g) in tetrahydrofuran (7.0 ml). After
stirring 1 hour, the reaction is transferred via cannnula into a solution of 1-(3-
N-ethylamino-2-pyridinyl)piperazine (0.70 g) in tetrahydrofuran (7 ml) at -
12°C (ice/acetone bath). The reaction is stirred at -10°C for 30 minutes, then
slowly warmed to 20°-25°C and stirred a further 18 hours. After diluting with ether (60 ml), the mixture is washed with saturated aqueous sodium bicarbonate (70 ml), saline (70 ml) and dried over anhydrous sodium sulfate.
The mixture is concentrated under reduced pressure to a residue which is
purified by flash chromatography (2 cm x 20 cm) eluting with
methanol/chloroform (2/98) to give the title compound, mp 153°-154°C.
1-[5-Methoxyindolyl-2-carbonyl]-4-[3-(ethylamino)-2-pyridinyl]piperazine
hydrochloride:
1-(Ethyl)-3-(dimethylaminopropyl)carbodiimide (1.25 g) is added to a solution
of 1-(3-ethyl-2-pyridinyl)piperazine (1.12 g) in THF (15 ml). The reaction is
stirred at 20°-25°C for 3 hr, then it is dissolved in chloroform (50 ml) and
extracted with saturated aqueous sodium bicarbonate, saline, dried over
anhydrous sodium sulfate and concentrated under reduced pressure.
Purification by flash column chromatography (200 g silica) eluting with ethyl
acetate/hexane (50/50), the appropriate fractions are pooled and
concentrated to give the title compound, The product is dissolved in methanol
(150 ml) with heating, cooled to 20°-25°C and chlorotrimethylsilane (4.70
mmol) is added. The mixture is concentrated to half-volume, ether is added
until cloudy and the flask is stored at 0°C overnight. Filtration gives the
hydrochloride salt, MP: 194°-195°C.
The mesylate salt is formed by dissolving the free base in methanol and by
adding methanesulfonic acid (1 eq). The solution is diluted with diethyl ether
until the salt crystallizes out of solution. The crystals are collected and dried
to afford the mesyl salt of the title compound, MP: 215°-216°C. | | Therapeutic Function | Antiviral |
| | atevirdine Preparation Products And Raw materials |
|