|
|
| | Calcium phosphate tribasic Basic information |
| Product Name: | Calcium phosphate tribasic | | Synonyms: | Hydroxyapatite nanopowder, <200 nM particle size (BET), >=97%, synthetic;Hydroxyapatite reagent grade, powder, synthetic;Hydroxyapatite synthetic, >=99.995% trace metals basis;Calcium phosphate tribasic, tribasic;calciumhydroxidephosphate(ca5(oh)(po4)3);PENTA-CALCIUM HYDROXIDE TRIPHOSPHATE;TERT-CALCIUM PHOSPHATE;hydroxyapatite, nanopowder | | CAS: | 12167-74-7 | | MF: | 5Ca.3O4P.HO | | MW: | 502.31 | | EINECS: | 235-330-6 | | Product Categories: | Materials Science;Nanoparticles: Oxides;Nanopowders and Nanoparticle Dispersions;Nitrides;20: Ca;Materials Science;Nanomaterials;NanomaterialsMaterials Science;Nanoparticles: Oxides, Nitrides, and Other CeramicsNanomaterials;Nanopowders and Nanoparticle Dispersions;New Products for Materials Research and Engineering;Inorganics;Ca (Calcium) Compounds;Classes of Metal Compounds;Typical Metal Compounds;20: Ca;and Other Ceramics;Biocompatible Ceramics;Biomaterials | | Mol File: | 12167-74-7.mol | 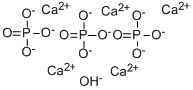 |
| | Calcium phosphate tribasic Chemical Properties |
| | Calcium phosphate tribasic Usage And Synthesis |
| Chemical Properties | The PhEur 6.4 states that tribasic calcium phosphate consists of a mixture of calcium phosphates. It contains not less than 35.0% and not more than the equivalent of 40.0% of calcium. The USP32– NF27 specifies that tribasic calcium phosphate consists of variable mixtures of calcium phosphates having the approximate composition 10CaO·3P2O5·H2O. This corresponds to a molecular formula of Ca5(OH)(PO4)3 or Ca10(OH)2(PO4)6.
Tribasic calcium phosphate is a white, odorless and tasteless powder. col hexagonal crystal(s) [CRC10] | | Uses | Si-substituted hydroxyapatite (Si-HAp) nanopowder can be incorporated in biodegradable polymer composites or deposited on biocompatible surfaces similar to pure HAp nanopowder (cat. no. 677418). Biological activity of HAp was shown to be enhanced by substitution of silicon ions into a fraction of HAp lattice phosphate sites.1,2 | | Uses | Calcium phosphate tribasic (C3161) is plant cell culture tested (0.2 mg/ml) and is appropriate for use in plant cell culture experiments. Calcium phosphate tribasic is utilized to engineer new biomaterials for applications such as bone grafts and fillers. | | Production Methods | Tribasic calcium phosphate occurs naturally as the minerals
hydroxylapatite, voelicherite, and whitlockite. Commercially, it is
prepared by treating phosphate-containing rock with sulfuric acid.
Tribasic calcium phosphate powder is then precipitated by the
addition of calcium hydroxide. Tribasic calcium phosphate is
alternatively prepared by treating calcium hydroxide from limestone
with purified phosphoric acid. It may also be obtained from
calcined animal bones. Some tribasic calcium phosphate products
may be prepared in coarser, directly compressible forms by
granulating the powder using roller compaction or spray drying. | | Application | Calcium phosphate tribasic is the mineral component of bones and teeth; it has been used therapeutically as a prosthetic aid and in the prevention and treatment of osteoporosis. Employed in tissue replacement for repairing bony defects and acts as drug carrier for drug delivery to the bone. Hydroxyapatite nanopowders, incorporated in biodegradable polymer composites or deposited on biocompatible substrates, have been shown to promote adhesions and proliferation of bone-forming (osteoblast) cells. It is also the starting material for the production of phosphoric acid and fertilizers. | | General Description | Hydroxyapatite is a key inorganic phosphate that has chemical and structural resemblance to bone. HAp is a popular biomaterial which finds use as a bone or teeth replacement and repair, hard tissue repair. | | Flammability and Explosibility | Non flammable | | Pharmaceutical Applications | Tribasic calcium phosphate is widely used as a capsule diluent and
tablet filler/binder in either direct-compression or wet-granulation
processes. The primary bonding mechanism in compaction is plastic
deformation. As with dibasic calcium phosphate, a lubricant and a
disintegrant should usually be incorporated in capsule or tablet
formulations that include tribasic calcium phosphate. In some cases
tribasic calcium phosphate has been used as a disintegrant.It is
most widely used in vitamin and mineral preparations as a filler
and as a binder. It is a source of both calcium and phosphorus, the
two main osteogenic minerals for bone health. The bioavailability
of the calcium is well known to be improved by the presence of
cholecalciferol. Recent research reports that combinations of
tribasic calcium phosphate and vitamin D3 are a cost-effective
advance in bone fracture prevention.
In food applications, tribasic calcium phosphate powder is
widely used as an anticaking agent. | | Safety | Tribasic calcium phosphate is widely used in oral pharmaceutical
formulations and food products, and is generally regarded as
nontoxic and nonirritant at the levels employed as a pharmaceutical
excipient.
Ingestion or inhalation of excessive quantities may result in the
deposition of tribasic calcium phosphate crystals in tissues. These
crystals may lead to inflammation and cause tissue lesions in the
areas of deposition.
Oral ingestion of very large quantities of tribasic calcium
phosphate may cause abdominal discomfort such as nausea and
vomiting.
No teratogenic effects were found in chicken embryos exposed to
a dose of 2.5 mg of tribasic calcium phosphate.
LD50 (rat, oral): >1 g/kg | | storage | Tribasic calcium phosphate is a chemically stable material, and is
also not liable to cake during storage.
The bulk material should be stored in a well-closed container in a
cool, dry place. | | Incompatibilities | All calcium salts are incompatible with tetracycline antibiotics.
Tribasic calcium phosphate is incompatible with tocopheryl acetate
(but not tocopheryl succinate). Tribasic calcium phosphate may
form sparingly soluble phosphates with hormones. | | Regulatory Status | GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (oral capsules
and tablets). Included in nonparenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients. |
| | Calcium phosphate tribasic Preparation Products And Raw materials |
|