- Huperzine a
-

- $150.00 / 1kg
-
2025-04-25
- CAS:102518-79-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500kg
- (-)-Huperzine A
-

- $0.00 / 1kg
-
2025-04-25
- CAS:102518-79-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500
- Huperzia Serrata extract
-

- $0.00 / 1KG
-
2025-04-25
- CAS:102518-79-6
- Min. Order: 1KG
- Purity: 98% up, High Density
- Supply Ability: 20 tons
|
| Product Name: | (-)-Huperzine A | | Synonyms: | Huperzine-A1-5%;(5r-(5-alpha,9-beta,11e))-ydro-7-methyl;5,9-methanocycloocta(b)pyridin-2(1h)-one,5-amino-11-ethylidene-5,6,9,10-tetrah;HUPERIZINE;(-)-HUPERZINE A FROM HUPERZIA SERRATA;HuperziaSerrateP.E120786-18-7/;(5R,9R,E)-5-AMino-11-ethylidene-7-Methyl-5,6,9,10-tetrahydro-5,9-Methanocycloocta[b]pyridin-2(1H)-one;(-)-Huperzine A (HupA) | | CAS: | 102518-79-6 | | MF: | C15H18N2O | | MW: | 242.32 | | EINECS: | 600-320-6 | | Product Categories: | Inhibitor;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Nutritional Ingredients;reference substance;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Acetylcholine receptor;API;Alkaloids;102518-79-6 | | Mol File: | 102518-79-6.mol | 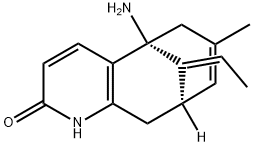 |
| | (-)-Huperzine A Chemical Properties |
| Melting point | 211-216oC | | alpha | D -147° (c = 0.36 in CH3OH) (Ayer); D24.5 -150.4° (c = 0.498 in MeOH) (Liu) | | Boiling point | 505.0±50.0 °C(Predicted) | | density | 1.20±0.1 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | insoluble in H2O; ≥12.12 mg/mL in DMSO; ≥23.13 mg/mL in EtOH | | pka | 12.25±0.60(Predicted) | | form | Solid | | color | White to Almost white | | optical activity | [α]/D -153±5°, c = 0.5 in methanol | | InChI | InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | | InChIKey | ZRJBHWIHUMBLCN-YQEJDHNASA-N | | SMILES | C12C[C@@]3([H])/C(=C\C)/[C@@](N)(C=1C=CC(=O)N2)CC(C)=C3 | | LogP | 0.833 (est) | | CAS DataBase Reference | 102518-79-6(CAS DataBase Reference) |
| | (-)-Huperzine A Usage And Synthesis |
| Huperzia serrata extract | Huperzine A is a natural plant alkaloid that extracted from the Chinese medicine Huperzia serrata under the genus Huperzia. It is a potent, revisable and highly selective second generation of acetylcholinesterase inhibitors, with the appearance of yellow to white crystalline powder, and is freely soluble in chloroform, soluble in methanol and ethanol, slightly soluble in water, with high lipid solubility. It is a small molecule that can well penetrate the blood brain barrier, and after entering the central nervous, it distributes more in the brain's frontal lobe, temporal lobe, hippocampus and areas that are closely related to learning and memory. It has a strong inhibitory effect on acetylcholinesterase (AchE) at a low dosage, significantly increasing the content of acetylcholine (Ach) in neural synaptic cleft in the distribution area, thus enhancing neuronal excitatory transmission, strengthening the excitement of learning and memory in the brain, thereby with the function of improving cognitive function, enhancing memory retention and promoting memory reproduction. It is currently the most successful development of Alzheimer's disease (senile dementia) drugs.
The above information is edited by the Chemicalbook of Cheng Jingmin.
| | Indications | Huperzine A is a potent reversible cholinesterase inhibitor, stronger than physostigmine, neostigmine and Tacrine. When used for myasthenia gravis, the effective rate reaches to 99%.
Clinical trials show that the product is suitable for benign memory disorders. It can improve patients’ ability in directed memory, associative learning, image memory, meaningless figure recognition and portrait retrieval,and it also can enhance normal people’s ability in learning and memory. This product also can improve memory disorders that caused by dementia and organic pathologic changes in brain.
Clinically, Huperzine A is applicable to the treatment of the following symptoms:
1. for the treatment and improvement of memory dysfunction in elder age, improving memory association function; for the memory deterioration caused by excessive use of the brain, improving work efficiency;
2. for memory function deterioration associated with neurasthenia;
3. for memory deterioration caused by cerebral vascular disorder;
4. for memory improvement of Alzheimer's disease, and it has significant effects on improving and restoring the patient's cognitive ability, memory function and abnormal emotion behaviors;
5. for the treatment of myasthenia gravis;
6. for improvement of disturbance of association, low cognitive function, memory deterioration that associated with schizophrenia;
7. for improvement of memory dysfunction associated with a variety of brain diseases and physical disorders.
| | Side effects | Skin hives, abdominal pain, salivation, muscle twitching, diarrhea, and insomnia, but not common.
Overdose can cause dizziness, nausea, gastrointestinal discomfort, chest tightness, fatigue, bradycardia and other reactions. The symptoms usually disappear on their own, and relief or disappear after stopping or reduction of the product when reacting significantly.
| | Inhibition | Huperzine A has selective inhibition for true cholinesterase, and the inhibition strength is thousands of times stronger than pseudocholinesterase. The suppression mode is mixed inhibition of competitive and noncompetitive, with significant differences with pure competitive inhibitors. This product crosses the blood-brain barrier into the central nervous easily, having both central and peripheral therapeutic effects; it has a long effective time; it is well absorbed from the gastrointestinal tract; it has large safety index; it has good stability.
The comparison result of inhibition intensity of different drugs to acetylcholinesterase (AChE): Huperzine A> physostigmine> neostigmine> Huperzine B> galantamine> galanin.
The inhibition strength to human butyrylcholinesterase (BuChE): physostigmine> neostigmine> Huperzine A> Huperzine B.
The functions of this product in both strengthening muscle contraction amplitude that induced by indirect electrical nerve stimulation and enhancing memory in rats are stronger than physostigmine, but its toxicity is lower than physostigmine, and it has longer duration of effectiveness.
| | Uses | Huperzine A is a new drug for the treatment of benign memory disorders that can effectively prevent cerebral neurasthenia in the middle-aged and elderly, restore cranial nerve function, and activate cranial neurotransmitters. Huperzine A can not only inhibite the activity of cholinesterase, but also improve cognitive function and ability of learning and memory through a variety of pharmacological mechanisms like effecting the system of free radicals, reducing expression levels of somatostatin, intracellular [Ca2+], glutamic acid content and increase calmodulin (CaM) and calmodulin messenger RNA (CaM mRNA). | | Description | Huperzine A is obtained from Huperzia serrata, which is the perennial fern. It
shows activities in antipyretic, hemostasis, and dehumidification and is used for the
treatment in folk of pneumonia, lung abscess, hematemesis, hematochezia, traumatic injury, etc. | | Chemical Properties | Pale Brown Solid | | Physical properties | Appearance: white crystalline powder. Bitter with hygroscopicity. Solubility: easily
soluble in chloroform, soluble in methanol and ethanol, and slightly soluble in
water. Melting point: 211–216?°C. | | History | In the 1980s, Chinese scholars isolated huperzine A from Lycopodiales,
Huperziaceae, Phlegmariurus fordii, and Huperzia serrata (Thunb.) Trevis.
At present, about 120 chemical components have been isolated and identified from
the plant, including 90 lycopodium alkaloids and 32 lycopodium triterpenes.
Huperzine A has the most potent inhibition on acetylcholinesterase activity, followed by huperzine B and 6β-hydroxy huperzine A.?These three compounds belong
to lycodine-type lycopodium alkaloids. The full synthesis of
huperzine A is complex and costly. Therefore, it is a focus to develop biotransformation or semisynthesis with other alkaloids as lead compounds on the basis of the
intrinsic relationship among different kinds of alkaloids
After the determination of chemical structure of huperzine A in 1986, it was
found to be the same alkaloid as selagine separated from Lycopodium selago by
Valenta in the 1960s, so it was classified as lycodine-type alkaloid. Huperzine A is
a potent reversible inhibitor of AChE, and its ability to improve learning and memory has been validated in animal models. It was approved for treatment in Alzheimer’s
disease (AD) in China in 1996
Due to the high cost of extraction of huperzine A, research on its chemical synthesis has been the focus at home and abroad since 1986. It has been found that the
chiral structure of huperzine A is essential for its biological activity; the inhibitory
activity on AChE of natural products (-)?- huperzine A is twice as its raceme and
38–50 times as its enantiomer (+)?- huperzine A which is not a natural product, so
the chemical synthesis of natural product (-)?- huperzine A has received extensive
attention. The chemical preparation method of (-)?- huperzine A can be divided into
asymmetric synthesis and raceme separation and is limited to a small amount preparation in laboratory for the high cost.
Considering the difficulty of realizing unique bridge ring and amino structure of
(-)?- huperzine A and achieving its full synthesis and structural modification, scientists are trying to synthesize analogues of huperzine A with simple structure and
AchE inhibitory activity. It was found that the activity of spearmint huperzine A was
similar to that of huperzine A, with improved selectivity and poor chemical stability.
It was modified structurally to obtain ZT-1 . | | Uses | Huperzine A is a potential therapeutic agent for Alzheimer disease that reversible alkaloid inhibitor of AChE which crosses the blood-brain barrier. It reduces cell death induced by glutamate in primary cultures derived from forebrain, hippocampus, cortex and cere.
In China, it is approved for use in the treatment of Alzheimer’s disease (AD). Huperzine A was classified as a dietary supplement by the FDA in 1997. As a nutraceutical, it is available in American health food stores or via the Internet, labeled as a memory aid. | | Indications | Huperzine A is purified from Chinese club moss and has been traditionally used in China for the treatment of swelling, fever, inflammation, blood disorders, and schizophrenia. It was mainly applied to age-related memory dysfunction and Alzheimer and has a good effect on improving memory function. It can be used to treat various types of Alzheimer's disease and also myasthenia gravis. | | Preparation | (-)-Huperzine A, a Lyco-podium alkaloid isolated in 1986 from the club moss Huperzia serrata, has drawn considerable attention after it was revealed to be a potent, selective, and reversible AChE inhibitor.
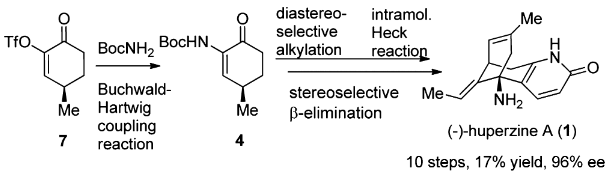
The total synthesis of Lycopodium alkaloid (-)-huperzine A has been accomplished in 10 steps with 17% overall yield from commercially abundant (R)-pulegone. The synthetic route features an efficient synthesis of 4 via a Buchwald–Hartwig coupling reaction, a dianion-mediated highly stereoselective alkylation of 4, and a rare example of an intramolecular Heck reaction of an enamine-type substrate. The stereoselective β-elimination and the accompanying Wagner–Meerwein rearrangement are of particular interest.
[1] RUI DING Guo Q L Bing Feng Sun*. An Efficient Total Synthesis of (-)-Huperzine A [J]. Organic Letters, 2012, 14 17: 4446-4449. DOI:10.1021/ol301951r.
[2] RUI DING. Divergent Total Synthesis of the Lycopodium Alkaloids Huperzine A, Huperzine B, and Huperzine U[J]. The Journal of Organic Chemistry, 2013, 79 1: 240-250. DOI:10.1021/jo402419h.
[3] TUN M K M, WüSTMANN D J, HERZON S B. A robust and scalable synthesis of the potent neuroprotective agent (-)-huperzine A [J]. Chemical Science, 2011, 11: 2251-2253. DOI:10.1039/C1SC00455G. | | Definition | ChEBI: Huperzine A is a sesquiterpene alkaloid isolated from a club moss Huperzia serrata that has been shown to exhibit neuroprotective activity. It is also an effective inhibitor of acetylcholinesterase and has attracted interest as a therapeutic candidate for Alzheimer's disease. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, a neuroprotective agent, a plant metabolite and a nootropic agent. It is a sesquiterpene alkaloid, a pyridone, a primary amino compound and an organic heterotricyclic compound. It is a conjugate base of a huperzine A(1+). | | Pharmacology | Huperzine A has the ability to enhance learning and memory, improve spatial memory, and can be used for age-related dementia, vascular dementia, and other neurodegenerative diseases. Compared with the current anti-AD drugs, huperzine A can go through the blood-brain barrier, with a high oral bioavailability and longer time
inhibition on AChE.
As a highly selective AChE reversible inhibitor, huperzine A can inhibit AChE,
reduce acetylcholine hydrolysis, and improve the level of acetylcholine in the synaptic gap. This inhibition is reversible, lasts for a long time, shows no drug dependence if repeated administration, and does not induce significant liver toxicity.
X-ray diffraction results show that the direct binding of huperzine A to AChE active
sites inhibits the binding of AChE to its substrate.
In addition to the potent inhibition on AChE, huperzine A only shows a weak
inhibitory effect on the butyrylcholinesterase; also protects neurons by inhibiting
oxidative stress, reducing somatostatin, reducing the content of glutamate, decreasing the increased intracellular calcium, and inhibiting neuronal apoptosis; further
improves AD-related cognitive function and reduces the symptoms of AD patients. | | Clinical Use | Since 1994, huperzine A has been approved for clinical use in improving memory
and cognitive impairment in old patients with memory loss and dementia. A large
number of domestic clinical studies have found that huperzine A shows therapeutic
effect on learning and cognitive dysfunction of vascular dementia, mental retardation, and schizophrenia patients with mild adverse reactions. | | target | AChE | NGF | NMDA receptor | | storage | +4°C (desiccate) |
| | (-)-Huperzine A Preparation Products And Raw materials |
|