- POLYACRYLONITRILE
-

- $6.00 / 1kg
-
2025-04-03
- CAS:25014-41-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- Polyacrylonitrile
-

- $75.00 / 1kg
-
2025-04-02
- CAS:25014-41-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Crospovidone
-

- $5.00 / 1KG
-
2025-03-28
- CAS:25014-41-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS
Related articles - What is Polyacrylonitrile?
- Polyacrylonitrile (C3H3N)n, is a high molecular compound, which is obtained from monomer acrylonitrile through radical polymer....
- Aug 24,2021
|
| | Polyacrylonitrile Chemical Properties |
| Melting point | 317 °C | | density | 1.184 g/mL at 25 °C (lit.) | | Tg | 125 | | refractive index | n20/D 1.514 | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMF (Slightly) | | form | Solid | | color | White to Off-White | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChI | InChI=1S/C3H3N/c1-2-3-4/h2H,1H2 | | InChIKey | NLHHRLWOUZZQLW-UHFFFAOYSA-N | | SMILES | C(#N)C=C | | EPA Substance Registry System | Polyacrylonitrile (25014-41-9) |
| Safety Statements | 24/25 | | WGK Germany | 3 | | RTECS | AT6977900 | | TSCA | TSCA listed | | HS Code | 39069090 | | Hazardous Substances Data | 25014-41-9(Hazardous Substances Data) | | Toxicity | rat,LC,inhalation,> 25gm/m3 (25000mg/m3),Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 6(6), Pg. 54, 1962. |
| | Polyacrylonitrile Usage And Synthesis |
| Description | Polyacrylonitrile is a kind of synthetic resin manufactured through the free radical polymerization of acrylonitrile. It belongs to the acrylic resin family. It is thermoplastic, and is resistant to most solvents and chemicals because of the strong chemical bonds formed between the nitrile groups. It is a kind of versatile polymer which can be used to produce many kinds of products such as fibers for textiles, ultra filtration membrane, carbon fiber and hollow fibers for reverse osmosis. Its homopolymer can also be used as fibers in outdoor awning, sails for yachts, and fiber-reinforced concrete. It can also be used for production of inherently flame resistant (FR) fabrics. | | Chemical Properties | Polyacrylonitrile(PAN) is a derivative of acrylic acid where the carboxylic acid group is replaced by the related nitrile group and presents the molecular formula (C3H3N)n.
PAN is a semi-crystalline polymer and though it is a thermoplastic, it does not melt under normal conditions. Its degradation occurs before it melts and its melting is usually observed at above 300°C. When this polymer is heated up to 200°C, it can be transform into a rigid structure with a release of energy, an occurrence referred to as cyclization.Above the cyclization temperature, this polymer undergoes oxidation, a scenario that confers non-flammability on it. If PAN is heated to above 1000°C under inert atmosphere, it gives a product with a percentage of carbon greater than 90%.This property makes it usable in carbon fiber production.
Some major characteristic properties that render PAN special compared to other polymers include the fact that it is the most resistant polymer to degradation by ultraviolet rays and sunlight and it is highly chemically unreactive and resistant to most organic acids and solvents. In addition, this polymer can be attacked only by concentrated solutions of alkalis and highly polar liquids.PAN in the form of acrylic fiber is resistant to breakages, is soft and comfortable, possesses high thermal insulation properties, and it does not melt maintaining its morphological structure when it is heated.This last property of PAN is utilized for the production of insulation fibers, flame retardant fibers, carbon fiber and blankets for filtration of hot gases. | | Uses | Due to its special properties such as stability to UV degradation, low density, high strength and modulus of elasticity, thermal stability, non-fusibility and chemical resistance, Polyacrylonitrile is an important polymer for a wide range of applications.Thus, it is used in textile applications due to its wool-like characteristics, in the making of carbon fiber, filtration membranes, fibers for cement reinforcement, sails for yachts, awning fabrics in outdoor applications, specialist fibers for acoustic and thermal insulation, anti-flame fibers and in the production of felts for hot air filtration systems.
Copolymers containing polyacrylonitrile, which are flame-retardant, are used asfibers to make knitted clothing, like socks and sweaters, as well as outdoor productslike tents. Poly(acrylonitrile-co-butadiene-co--styrene)(ABS) and Poly(styrene-co-acrylonitrile)(SAN) are used as plastics. ABS is a lightweight and very strong polymer. It is so light and strong enough to be used in the production of automobile body parts.Thus, this material render automobiles'lighter using less fuel and thus polluting less.
The strength of ABS emanates from the nitrile groups that are present in its acrylonitrile units. The nitrile groups are very polar; hence, they can be attracted to one another. This occurrence permits opposite charges on the nitrile groups to stabilize one another. This strong attraction holds ABS chains very tightly, thus, itmakes the material very strong.Furthermore, the rubbery polybutadiene present in it makes ABS a tough polymer. | | References | https://www.britannica.com/science/polyacrylonitrile
https://en.wikipedia.org/wiki/Polyacrylonitrile
| | Chemical Properties | white chalk-like solid | | Uses | Polyacrylonitrile (PAN) is used as polymeric carbon precursor to form carbon fibers, electrospun activated carbon materials having meso-macro pores, carbon black additives.These are consecutively used in hydrogen storage, EMI shielding, electrochemistry, separation processes. It may find applications in PAN based single walled carbon nanotube composites. | | Definition | ChEBI: A macromolecule composed of repeating cyanoethylene units. | | Preparation | Approximately 70% of the commercial output of acrylonitrile is polymerized
(with minor amounts of comonomers) to give polymers which are used for
textile fibres:
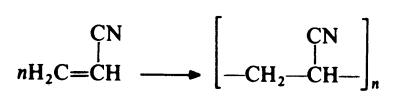
The most important methods for the preparation of polyacrylonitrile are
solution polymerization and suspension polymerization. The former method
is particularly convenient, since when a solvent for the polymer is used, the resulting solution may be utilized directly for fibre spinning. Concentrated
aqueous solutions of inorganic salts such as calcium thiocyanate, sodium
perchlorate and zinc chloride make suitable solvents; suitable organic solvents include dimethylacetamide, dimethylformamide and dimethylsulphox�ide. Emulsion polymerization suffers from the disadvantage that the monomer has appreciable water-solubility and the formation of polymer in the
aqueous phase can lead to coagulation of the latex. This tendency is reduced
by the addition of ethylene dichloride to the system.
Fibres prepared from straight polyacrylonitrile are difficult to dye and, in
order to improve dyeability, commercial fibres invariably contain a minor
proportion (about 10%) of one or two comonomers such as methylmeth�acrylate, vinyl acetate and 2-vinylpyridine.
The average molecular weight (Mw) of commercial polyacrylonitrile is
generally in the range 80000-170000.
In polyacrylonitrile appreciable electrostatic forces occur between the
dipoles of adjacent nitrile groups on the same polymer molecule. This
intramolecular interaction restricts bond rotation and leads to a stiff chain.
As a result, polyacrylonitrile has a very high crystalline melting point (317??C)
and is soluble in only a few solvents such as dimethylacetamide and dimethyl�formamide and in aqueous solutions of inorganic salts. Polyacrylonitrile
cannot be melt processed since extensive decomposition occurs before any
appreciable flow occurs and fibres are therefore spun from solution. In one
process, for example, a solution of the polymer in dimethylformamide is
extruded into a coagulating bath of glycerol and the fibre formed is drawn
and wound.
Polyacrylonitrile is unstable at elevated temperatures. On heating above about 200??C, polyacrylonitrile yields a red solid with
very little formation of volatile products. When the red residue is heated at
about 350??C there is produced a brittle black material of high thermal
stability. The first step in these changes consists of a nitrile polymerization
reaction whilst the second step involves aromatization to form a condensed
polypyridine ladder polymer:
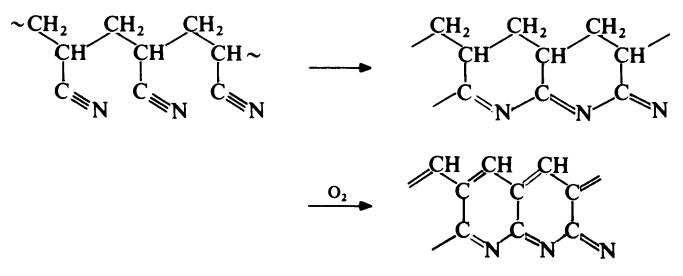
Continued heating at high temperatures (1500-3000??C) results in the elimination of all elements other than carbon to leave a carbon fibre with graphitic crystalline structure of great strength. Polyacrylonitrile fibres have become
the most important source for carbon fibres.
Polyacrylonitrile is hydrolysed by heating with concentrated aqueous
sodium hydroxide to poly(sodium acrylate). | | General Description | PAN molecule has strong polar nitrile groups.It is relatively insoluble in nature. PAN based carbon fibers possess very high strength compared to other polymeric precursors. When subjected to heat treatment, it can produce high carbon yield giving rise to thermally stable, highly oriented, graphite like molecular structure. Generating carbon-fiber from PAN based fiber is a combination of three processes-- namely stabilization, carbonization and graphitization. | | Solubility in organics | Adiponitrile, DMAC, DMF, DMSO | | Purification Methods | Precipitate it from dimethylformamide by addition of MeOH. |
| | Polyacrylonitrile Preparation Products And Raw materials |
|