|
|
| | N-(chloracetyl)methanesulfonamide Basic information |
| | N-(chloracetyl)methanesulfonamide Chemical Properties |
| Melting point | 114 - 116°C | | density | 1.474±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 3.43±0.40(Predicted) | | form | Solid | | color | White to Off-White | | InChI | InChI=1S/C3H6ClNO3S/c1-9(7,8)5-3(6)2-4/h2H2,1H3,(H,5,6) | | InChIKey | BKLFBPWLPOUGTM-UHFFFAOYSA-N | | SMILES | C(NS(C)(=O)=O)(=O)CCl |
| | N-(chloracetyl)methanesulfonamide Usage And Synthesis |
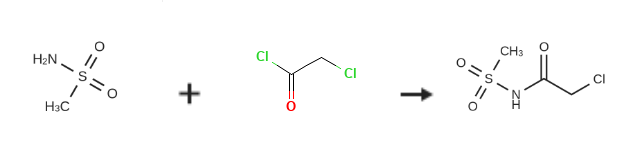
| Uses | 2-Chloro-N-(methylsulfonyl)acetamide acts as a reagent for the preparation of piperazinylquinolonecarboxamides as antivirals. Solid phase preparation of peptidines, glycine-amidine-based oligomers. | | storage | Inert atmosphere,Store in freezer, under -20℃. | | References | N-(chloracetyl)methanesulfonamide is prepared by the reaction of methanesulfonamide and chloroacetyl chloride. The specific synthesis steps are as follows:
In a 2-liter reaction flask, 1 liter of ethyl acetate and 66 grams of methylsulfonamide were added, and 109 grams of chloroacetyl chloride was gradually added; the temperature was gradually increased to 65C for 12 hours until the end of the reaction.The reaction solution gradually cooled to 0 degree, and a large amount of white solid precipitated; it was filtered and dried to obtain 112 g of solid SLP-10b (X=Cl).Yield: 94%.H NMR (400MHz, CDCl3): delta 4.02 (s, 2H), 3.28s, 3H)ESI/MS+(m/z):171.
 |
| | N-(chloracetyl)methanesulfonamide Preparation Products And Raw materials |
|