- Clorprenaline
-

- $50.00 / 1KG
-
2025-03-21
- CAS:3811-25-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000KG
- Clorprenaline
-
- $30.00 / 2mg
-
2024-11-19
- CAS:3811-25-4
- Min. Order:
- Purity:
- Supply Ability: 10g
- Clorprenaline
-
- $1.00 / 1KG
-
2019-08-06
- CAS:3811-25-4
- Min. Order: 1KG
- Purity: 95%-99%
- Supply Ability: 2000kg
|
| | Clorprenaline Basic information |
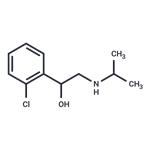
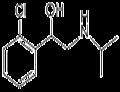
| Product Name: | Clorprenaline | | Synonyms: | 1-(2-Chlorophenyl)-2-(isopropylamino)ethanol;Benzenemethanol, 2-chloro-alpha-[[(1-methylethyl)amino]methyl]-;Isoprophenamineisoprofenamine;o-Chloro-alpha-[(isopropylamino)methyl]benzyl alcohol;CLORPRENALINE;Cloroprenaline;Isoprophenamine;Chlorprenaline | | CAS: | 3811-25-4 | | MF: | C11H16ClNO | | MW: | 213.7 | | EINECS: | 223-291-8 | | Product Categories: | | | Mol File: | 3811-25-4.mol |  |
| | Clorprenaline Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 22-36 | | Safety Statements | 26 | | WGK Germany | 3 | | HS Code | 29221990 |
| | Clorprenaline Usage And Synthesis |
| Originator | Asthone,Eisai,Japan,1970 | | Uses | Bronchodilator. | | Uses | Clorprenaline is a beta-2 adrenergic agonist with bronchodilatory activity. | | Definition | ChEBI: 1-(2-chlorophenyl)-2-isopropylaminoethanol is a member of the class of monochlorobenzenes that is chlorobenzene which is substituted by a 1-hydroxy-2-[(propan-2-yl)amino]ethyl group at position 2. It is a member of monochlorobenzenes, a member of ethanolamines and a secondary amino compound. | | Manufacturing Process | To a solution of 279 g of o-chloroacetophenone in 2 liters of anhydrous diethyl
ether were added about 3 g of dibenzoyl peroxide. 5 g of bromine were added
to the resulting solution, and after 3 minutes, the color of bromine had been
discharged, indicating that the formation of ω-bromo-o-chloroacetophenone
had been initiated. A further amount of 288 g of bromine was added dropwise
to the reaction mixture over a 1.5 hour interval. After the addition of the
bromine had been completed, the reaction mixture was stirred for one-half
hour and poured over about 1 kg of crushed ice.
After the ice had melted, the resulting aqueous and ethereal layers were
separated. The ethereal layer containing ω-bromo-o-chloroacetophenone was
washed with successive 500 ml quantities of water, 5% sodium carbonate
solution and again with water to remove the hydrogen bromide formed as a
by-product in the reaction. The ethereal layer was dehydrated by contacting
with anhydrous magnesium sulfate. The drying agent was removed
filtration and the ether was evaporated from the filtrate. The residue
remaining after the evaporation consisted of about 400 g of ω-bromo-o�chloroacetophenone.
A solution of 400 g of ω-bromo-o-chloroacetophenone in one liter of methanol
was cooled to about 25°C. A cold solution of 92.5 g of sodium borohydride in
one liter of methanol was added as rapidly as possible to this cooled solution
while maintaining the temperature below about 25°C. After the addition had
been completed, the reaction mixture was allowed to stand for 4 hours at
ambient room temperature, to complete the reduction of the keto group of the
ω-bromo-o-chloroacetophenone. The reaction mixture containing a mixture of
o-chlorophenyl ethylene-β-bromohydrin and o-chlorophenyl ethylene oxide
was then evaporated in vacuo at room temperature to a syrup which was
poured into about one liter of 5% hydrochloric acid to decompose any borate�alcohol complexes.
The two compounds were dissolved in diethyl ether by extracting the acidic
layer three times with successive 500 ml portions of diethyl ether. The
combined ether extracts were dried over anhydrous magnesium sulfate and
filtered, and the ether was removed by evaporation in vacuo. A residue
consisting of 400 g of a mixture of o-chlorophenyl ethylene-β-bromohydrin
and o-chlorophenyl ethylene oxide was obtained.
400 g of a mixture of o-clilorophenyl ethylene-β-bromohydrin and o�chlorophenyl ethylene oxide were dissolved in one liter of anhydrous ethanol.
To this solution was added a solution of 306 g of isopropylamine in one liter of
anhydrous ethanol. The reaction mixture was heated at refluxing temperature
for about 16 hours, thus forming N-[β-(o-chlorophenyl)-β-hydroxyethyl]-
isopropylamine. The solvent was removed in vacuo, and to the residue was
added a solution containing 200 ml of 12 N HCl in 2,500 ml of water.
The acidic solution was washed twice with 500 ml portions of ether which
were discarded. The acidic layer was then made basic by the addition of 250
ml of 5% (w/v) sodium hydroxide, thus liberating the free base of N-[β-(o�chlorophenyl)-β-hydroxyethyl]-isopropylamine. The free base was extracted
with two successive one liter portions of diethyl ether. The combined ether
extracts were dried over anhydrous magnesium sulfate, filtered and
concentrated in vacuo to remove all of the solvents. N-[β-(o-chlorophenyl)-β-
hydroxyethyl]-isopropylamine was thus obtained, according to US Patent
2,887,509.
The N-[β-(o-chlorophenyl)-β-hydroxyethyl]-isopropylamine obtained by the
foregoing procedure was dissolved in about 3 liters of ether and dry hydrogen
chloride gas was bubbled into the solution until it was saturated, whereupon
the hydrochloride salt of N-[β-(o-chlorophenyl)-β-(hydroxy)-
ethyl]isopropylamine precipitated. The salt was separated from the ether by
filtration, and was dissolved in two liters of anhydrous ethanol. The alcoholic
solution was decolorized with charcoal and filtered.
Three liters of anhydrous ether were added thereto and the N-[β-(o�chlorophenyl)-β-hydroxyethyl]-isopropylamine hydrochloride precipitated in
crystalline form as the monohydrate. The mixture was maintained at about
0°C for 40 hours and then filtered. The filter cake was washed with ether and
dried. About 209 g of N-[β-(o-chlorophenyl)-β-(hydroxy)-ethyl]isopropylamine
hydrochloride monohydrate, melting at about 163° to 164°C, were obtained
according to US Patent 2,816,059. | | Therapeutic Function | Bronchodilator |
| | Clorprenaline Preparation Products And Raw materials |
|