|
|
| | 2-(Perfluorobutyl)ethyl acrylate Basic information |
| Product Name: | 2-(Perfluorobutyl)ethyl acrylate | | Synonyms: | 1H,1H,2H,2H-Nonafluorohexyl Acrylate (stabilized with TBC);1H,1H,2H,2H-Nonafluorohexyl Acrylate (stabilized with MEHQ);Perfluorobutylethyl acrylate;2-Propenoic acid 3,3,4,4,5,5,6,6,6-nonafluorohexyl ester;3,3,4,4,5,5,6,6,6-Nonafluorohexyl acrylate;3,3,4,4,5,5,6,6,6-Nonafluorohexylacrylate;Acrylic acid 2-(nonafluorobutyl)ethyl ester;Acrylic acid 3,3,4,4,5,5,6,6,6-nonafluorohexyl ester | | CAS: | 52591-27-2 | | MF: | C9H7F9O2 | | MW: | 318.14 | | EINECS: | | | Product Categories: | Halogenated Heterocycles ,Isoquinolines ,Quinolines ,Quinaldines | | Mol File: | 52591-27-2.mol | 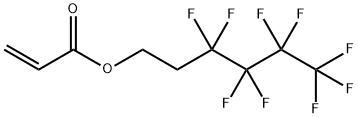 |
| | 2-(Perfluorobutyl)ethyl acrylate Chemical Properties |
| Boiling point | 164 °C | | density | 1.414 | | refractive index | 1.310 | | Fp | 52 °C | | storage temp. | Store at 2-8 ℃ | | solubility | Chloroform (Soluble), Ethyl Acetate (Slightly) | | form | clear liquid | | color | Colorless to Light yellow | | Specific Gravity | 1.440 | | EPA Substance Registry System | 1,1,2,2-Tetrahydroperfluorohexyl acrylate (52591-27-2) |
| RIDADR | UN 3272 3/PG III | | HazardClass | 3 | | PackingGroup | III | | HS Code | 2916120090 |
| | 2-(Perfluorobutyl)ethyl acrylate Usage And Synthesis |
| Uses | 2-(Perfluorobutyl)ethyl Acrylate can be used as an acrylic resin surface modifier with high water repellency and oil repellency for coating agents. It can also be used in a super concentrated emulsion method to prepare a solid sheet polycarboxylic acid water reducing agent. | | Application | 2-(Perfluorobutyl)ethyl acrylate has been used in a wide range of scientific research applications. It has been used to create polymers for biomedical applications, such as drug delivery systems and tissue engineering scaffolds. It has also been used to create polymers for industrial applications, such as coatings and adhesives. In addition, it has been used to create polymers for energy applications, such as fuel cells and solar cells. | | Synthesis | 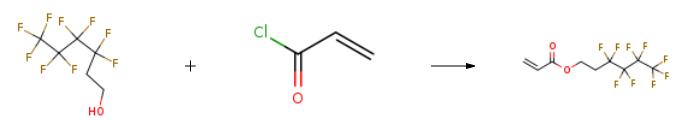
1H,1H,2H,2H-nonafluoro-1-hexanol (52.8 g) as fluoro alcohol and triethylamine (50 g) were dissolved in 500 ml of tetrahydrofuran. A solution of 18.1 g of acryloyl chloride as acid chloride in 100 ml of tetrahydrofuran was dropped in this solution over 2 hours while the mixture was being cooled in ice and stirred. After the completion of the dropping, the resulting white precipitate was filtrated and tetrahydrofuran and triethylamine were removed from the filtrate using a rotary evaporator. NMR measurement revealed that the resulting compound was 1H,1H,2H,2H-nonafluorohexyl acrylate. | | Research | Since the bioaccumulation of the degradation products of fluoropolymer with a long Rf side chain (C ≥ 8) has a potential environmental risk, it is necessary to design fluoropolymer coating with a short Rf side chain. Honda et al. studied the effect of α-substituent on the wetting behavior and molecular motion at the surfaces of poly{2-(perfluorobutyl)ethyl acrylate} [PFA-C4], poly{2-(perfluorobutyl)ethyl methacrylate} [PFMA-C4], poly{2-(perfluorobutyl)ethyl α-fluoroacrylate} [PFFA-C4], and poly{2-(perfluorobutyl)ethyl α-chloroacrylate} [PFClA-C4] films. In the case of PFA-Cy with long Rf groups [y ≥ 8], the mobility of the side chains is low at room temperature because of the crystallization of the Rf groups at the surface region[1].
| | References | [1] Koji Honda . "Effect of α-substituents on molecular motion and wetting behaviors of poly(fluoroalkyl acrylate) thin films with short fluoroalkyl side chains." Polymer 55 24 (2014): Pages 6303-6308. |
| | 2-(Perfluorobutyl)ethyl acrylate Preparation Products And Raw materials |
|