| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Clopirac
CAS:42779-82-8
Purity:98% Package:100 mg;5 mg;50 mg
|
| Company Name: |
Enamine
|
| Tel: |
(380) 44 537 32 18 |
| Email: |
enamine@enamine.net |
| Products Intro: |
|
| Company Name: |
Debye Scientific Corporation
|
| Tel: |
00852-2521 1836 |
| Email: |
|
| Products Intro: |
Product Name:2-(1-(4-chlorophenyl)-2,5-dimethyl-1H-pyrrol-3-yl)acetic acid
CAS:42779-82-8
|
|
| | 1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci Basic information |
| Product Name: | 1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci | | Synonyms: | BRL-13856;CP-172AP;2-(1-(4-chlorophenyl)-2,5-dimethyl-1H-pyrrol-3-yl)acetic acid;1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci;clopirac;1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticacid;1H-Pyrrole-3-acetic acid, 1-(4-chlorophenyl)-2,5-dimethyl- | | CAS: | 42779-82-8 | | MF: | C14H14ClNO2 | | MW: | 263.72 | | EINECS: | 255-938-5 | | Product Categories: | | | Mol File: | 42779-82-8.mol | 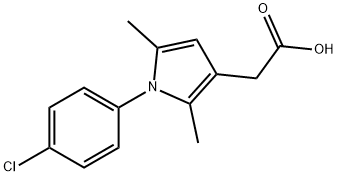 |
| | 1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci Chemical Properties |
| Boiling point | 429.1±45.0 °C(Predicted) | | density | 1.23±0.1 g/cm3(Predicted) | | pka | 4.38±0.10(Predicted) |
| | 1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci Usage And Synthesis |
| Originator | Nidran Cont. ,Pharma | | Uses | Anti-inflammatory. | | Definition | ChEBI: Clopirac is a member of pyrroles. | | Manufacturing Process | 2 Methods of producing of 1-p-chlorophenyl-2,5-dimethyl-3-pyrroleacetic acid
1. To 47.0 g (0.23 mol) of finely ground 1-p-chlorophenyl-2,5-dimethylpyrrole,
a solution of 47.5 ml of an aqueous solution of 40% dimethylamine, 57.5 ml
of acetic acid and 27.5 ml of 35% formaldehyde is slowly added while stirring.
The mixture is stirred overnight at room temperature and extraction is made
with 2 x 100 ml of ether. To the aqueous phase, 700 ml of 20% NaOH are
added and extraction is made with ether. The organic phase is dried on
MgSO4, filtered and evaporated. To the residue obtained, 60 ml of absolute
ethanol are added, then dropwise while stirring 34.1 g of methyl iodide. The
mixture is stirred for 1 h, then the precipitate obtained is filtered; 85.9 g
(yield: 92.5%) of methiodide of 1-p-chlorophenyl-2,5-dimethyl-3-
dimethylaminomethylpyrrole are thus obtained, melting point 197°-201°C
(dec).
To 166.0 g (0.41 mol) of the methiodide of 1-p-chlorophenyl-2,5-dimethyl-3-
dimethylaminomethylpyrrole in 600 ml of dimethylsulfoxide, 66.6 g of sodium
cyanide are added and the mixture is heated to 100°C with stirring and under
a nitrogen stream for 3.5 h. After cooling, the mixture is poured into 1500 ml
of water and extracted with ether. The ethereal phase is washed with water,
dried on MgSO4 and evaporated. The residue is vacuum stripped; 62.1 g of a
yellow oil are thus obtained, which rapidly solidifies and which is recrystallized
from aqueous methanol, 57.4 g (yield: 56%) of 1-p-chlorophenyl-2,5-
dimethyl-3-pyrrole acetonitrile are obtained, melting point 86°-88°C, boiling
point 158°-161°C (0.4 mm).
To 64.0 g of 1-p-chlorophenyl-2,5-dimethyl-3-pyrrole acetonitrile, 64.0 g of
KOH and 300 ml of ethanol are added and the mixture is refluxed for 15 h.
The alcohol is evaporated and one dilutes with 300 ml of water. The aqueous
phase is washed with ether, and then acidified with 20% HCl. The precipitated
obtained is filtered and one washes with petroleum ether and a minimum of
ether. 58.5 g (yield: 85%) of 1-p-chlorophenyl-2,5-dimethyl-3-pyrroleacetic
acid are thus obtained, melting point 99.5°-101°C.
2. To 9.5 g of 3-acetyl-2,5-hexanedione in 50 ml of benzene, 7.7 g of p�chloroaniline are added and the mixture is refluxed for 5 h with azeotropi
removal of the water formed. The excess solvent is evaporated and residue is
vacuum stripped: 12.0 g (yield: 80%) of 1-p-chlorophenyl-2,5-dimethyl-3-
acetylpyrrole as an orange oil which rapidly crystallizes are thus obtained,
boiling point 144°-146°C. Melting point of it 80°-81°C (recrystallization from
hexane).
To 5.0 g of the 1-p-chlorophenyl-2,5-dimethyl-3-acetylpyrrole, 0.7 g of sulfur
and 2.7 ml of morpholine are added and the mixture is refluxed for 5 h. Then,
20 ml of an aqueous solution of 20% are added and the mixture is refluxed
for 4 h. The mixture is washed with ether, acidified with 20% HCl, and then
extracted with ether. The organic phase is extracted with a 10% sodium
bicarbonate solution which is acidified with 5% HCl. The solid obtained is
filtered and recrystallized several times from a diethyl ether-pentane mixture.
1.1 g (yield: 20%) of 1-p-chlorophenyl-2,5-dimethyl-3-pyrroleacetic acid are
thus obtained, melting point 100°-103°C. | | Therapeutic Function | Antiinflammatory |
| | 1-(4-chlorophenyl)-2,5-dimethyl-1h-pyrrole-3-aceticaci Preparation Products And Raw materials |
|