| Company Name: |
Alta Scientific Co., Ltd.
|
| Tel: |
022-6537-8550 15522853686 |
| Email: |
sales@altasci.com.cn |
| Products Intro: |
Product Name:Triflupromazine
CAS:146-54-3
Purity:99% Package:10mg,100mg
|
| Company Name: |
Shaanxi Dideu Medichem Co. Ltd
|
| Tel: |
029-61856358 15229202216 |
| Email: |
1020@dideu.com |
| Products Intro: |
Product Name:triflupromazine
CAS:146-54-3
Purity:99%
|
triflupromazine manufacturers
- triflupromazine
-

- $8.00 / 1KG
-
2024-10-22
- CAS:146-54-3
- Min. Order: 1g
- Purity: Min98% HPLC/GC
- Supply Ability: g/kg/ton
|
| | triflupromazine Basic information |
| Product Name: | triflupromazine | | Synonyms: | dimethyl(3-(2-trifluoromethylphenothiazin-10-yl)propyl)amine;fluopromazine;triflupromazine;TRIFLUOROPROMAZINE;10-[3-(Dimethylamino)propyl]-2-(trifluoromethyl)-10H-phenothiazine;Trifluopromazine;Vetame;N,N-dimethyl-3-[2-(trifluoromethyl)phenothiazin-10-yl]propan-1-amine | | CAS: | 146-54-3 | | MF: | C18H19F3N2S | | MW: | 352.4170696 | | EINECS: | 2056736 | | Product Categories: | | | Mol File: | 146-54-3.mol | 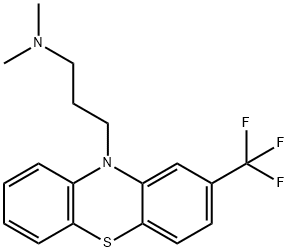 |
| | triflupromazine Chemical Properties |
| Melting point | 25°C | | Boiling point | bp0.7 176°; bp0.4 162-164° | | density | 1.2198 (estimate) | | refractive index | nD23 1.5780 | | pka | pKa 9.41(H2O (extrap)
t = 24±1
I~0.002) (Uncertain) | | Water Solubility | 1.762mg/L(24 ºC) |
| | triflupromazine Usage And Synthesis |
| Originator | Vesprin,Squibb,US,1957 | | Uses | Antipsychotic. | | Uses | In psychiatric practice, triflupromazine is used for psychomotor excitement in patients
with schizophrenia for paranoid and manic-depressive conditions, and for neurosis. | | Definition | ChEBI: A member of the class of phenothiazines that is 10H-phenothiazine having a trifluoromethyl subsitituent at the 2-position and a 3-(dimethylamino)propyl group at the N-10 position. | | Manufacturing Process | Approximately 3.8 grams of sodamide is freshly prepared from 2.25 grams of
sodium, 90 grams of liquid ammonia and a catalytic trace of ferric nitrate. The
ammonia is allowed to evaporate. A solution of 19.1 grams of 2-trifluoromethylphenothiazine (prepared by the Bernthsen thionation of 3-
trifluoromethyldiphenylamine) in 160 ml of dry benzene is added to the
reaction flask followed by 18 grams of 3-chloro-1-dimethylaminopropane. The
reaction mixture is heated at reflux for 20 hours. After washing the cooled
mixture with 130 ml of water, the organic layer is extracted with several
portions of dilute hydrochloric acid. The acid extracts are combined and
neutralized with ammonium hydroxide solution. The oily free base is extracted
into benzene and purified by distillation to give 19.6 grams of 10-(3'-
dimethylaminopropyl)-2-trifluoromethylphenothiazine, boiling point 177° to
181°C at 1 mm. The free base (7 grams) is converted to the hydrochloride
salt by reacting an alcoholic solution of the base with hydrogen chloride gas.
Evaporation of the volatiles in vacuo leaves an amorphous solid which is
recrystallized from ethanol/ether to pink crystals, MP 173° to 174°C, the
hydrochloride salt of the free base prepared above. | | Brand name | Vesprin
(Apothecon). | | Therapeutic Function | Tranquilizer | | Safety Profile | Poison by ingestion,
intravenous, and intraperitoneal routes.
Experimental reproductive effects. Mutation
data reported. When heated to
decomposition it emits very toxic fumes of
F-, NOx, and SOx. See also FLUORIDES. | | Synthesis | Triflupromazine, 2-trifluoromethyl-10-(3-dimethylaminopropyl) phe�nothiazine (6.1.3), also is synthesized by the alkylation of 2-trifluoromethylphenothiazine
using 3-dimethylaminopropylchloride in the presence of sodium amide [7–12]. |
| | triflupromazine Preparation Products And Raw materials |
|