
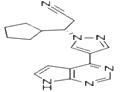
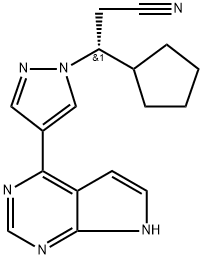
Ruxolitinib
| Price | $105 |
| Package | 1G |
| Min. Order: | 1G |
| Supply Ability: | customise |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Ruxolitinib | CAS No.: 941678-49-5 |
| EC-No.: 1312995-182-4 | Min. Order: 1G |
| Purity: 99% | Supply Ability: customise |
| Release date: 2019/07/06 |
WM0049
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1kg |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-29 | |
| $0.00/1Kg |
VIP1Y
|
airuikechemical co., ltd.
|
2024-04-09 | |
| $5.00/1KG |
VIP1Y
|
Henan Fengda Chemical Co., Ltd
|
2024-04-03 | |
| $0.00/1g |
VIP3Y
|
Senova Technology Co. Ltd.
|
2023-12-14 | |
| $160.00/1g |
VIP2Y
|
Guangzhou Tengyue Chemical Co., Ltd.
|
2023-11-14 | |
| $0.00/25KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-09-07 | |
| $10.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-08-17 | ||
| $0.00/1g |
VIP2Y
|
shandong perfect biotechnology co.ltd
|
2023-08-14 | |
| $0.00/1kg |
VIP2Y
|
Hangzhou ICH Biofarm Co., Ltd
|
2023-06-30 | |
| $0.00/1KG |
VIP2Y
|
Wuhan Senwayer Century Chemical Co.,Ltd
|
2022-11-07 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com





 China
China